How would you synthesize the following compounds from benzene? Assume that ortho and Para isomers can beseparated.
Question:
How would you synthesize the following compounds from benzene? Assume that ortho and Para isomers can beseparated.
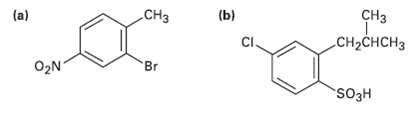
Transcribed Image Text:
(b) снз CH2CHCH3 (a) CHз Br O2N SO3H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (15 reviews)
Both of these syntheses test your ability to c...View the full answer

Answered By

Junaid ahmed
I am an English language professor with years of experience In Teaching English Language and Literature. I like to help people in the various difficult matter.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How would you synthesize the following compounds from Cyclohexanone? More than one step may berequired. () CH2 CH2Br (a) (c) o CH2C6H5 CH2CH2CO2H (d) (e) (f) O
-
How would you synthesize the following compounds from acetylene and any alkyl halides with four or fewer carbons? More than one step may herequired. (a) CH3CH2CH2C=CH (b) H2C%3CCH CH CH CHH2H3D (d)...
-
How would you synthesize the following compounds from Cyclohexanone? (a) 1-Methylcyclohexane (b) 2-Phenylcyclohexanone (c) cis-1, 2-Cyclohcxanediol (d) 1-Cyclohexylcyclohcxanol
-
Which of the following variable types can be used in a switch statement under some circumstances? (Choose three.) A. An enumerated type B. StringBuilder C. Byte D. Double E. var F. Exception.
-
During 2016, Jenny, age 14, lives in a household with her father, uncle, and grandmother. The household is maintained by the uncle. The parties, all of whom file separate returns, have AGI as...
-
In a study of graduate students who took the Graduate Record Exam (GRE), the Educational Testing Service reported a correlation of 0.37 between undergraduate grade point average (GPA) and the...
-
Prepare Hertog Companys journal entries to reflect the following transactions for the current year. May 7 Purchases 200 shares of Kraft stock as a short-term investment in available-for-sale...
-
Brite Lite Inc. manufactures light bulbs. Their purchasing policy requires that the purchasing agents place each quarters purchasing requirements out for bid. This is because the Purchasing...
-
Stanley Department Stores reported net income of $765,000 for the year ended December 31, 2020. The companys income tax rate is 40%. Additional Information: a. Common shares outstanding at Jan. 1,...
-
Listed below are amounts of arsenic in samples of brown rice from three different states. The amounts are in micrograms of arsenic and all samples have the same serving size. The data are from the...
-
Benzene and alkyl-substituted benzenes can be hydroxylated by reaction with H2O2 in the presence of an acidic catalyst. What is the structure of the reactive electrophile? Propose a mechanism for...
-
You know the mechanism of HBr addition to alkenes, and you know the effects of various substituent groups on aromatic substitution. Use this knowledge to predict which of the following two alkenes...
-
You are using the arbitrage pricing model to estimate the expected return on Bethlehem Steel, and have derived the following estimates for the factor betas and risk premia: a. Which risk factor is...
-
The following post-closing trial balance was drawn from the accounts of Spruce Timber Co. as of December 31, 2011. Transactions for 2012 1. Acquired an additional \(\$ 10,000\) cash from the issue of...
-
Bankers Trust (BT) was one of the most powerful and profitable banks in the world in the early 1990s. Under the stewardship of chairman Charles Sanford Jr., it had transformed itself from a staid...
-
Hammond Inc. experienced the following transactions for 2011, its first year of operations: 1. Issued common stock for \(\$ 80,000\) cash. CHECK FIGURES b. Net Income: \(\$ 62,520\) Total Assets:...
-
Following are the current prices and last years prices of a gallon of regular gas at a sample of 14 gas stations. Can you conclude that the median price is different now from what it was a year ago?...
-
A sample of nine men participated in a regular exercise program at a local gym. They were weighed both before and after the program. The results were as follows. Can you conclude that the median...
-
WHAT IS A COMPUTER NETWORK?
-
You have just begun your summer internship at Omni Instruments. The company supplies sterilized surgical instruments for physicians. To expand sales, Omni is considering paying a commission to its...
-
A power plant using a Rankine power generation cycle and steam operates at a temperature of 80 C in the condenser, a pressure of 2.5 MPa in the evaporator, and a maximum evaporator temperature of...
-
Provide names for thesecompounds: SH SH b) H OS e) CH3 SOCH,CH,CH, d)
-
Draw the structures for these compounds: (a) 2-Butanethiol (b) Benzenethiol (c) Isopropyl methanesulfonate (d) p-Bromobenzenesulfonic acid (e) Phenyl trichloromethyl sulfide
-
Provide systematic names for these compounds: NH2 ) H,, b) a) CO,H Leucine (an amino acid) CH OCH3 d) H. NHCH, CN h) g) CH,CCH,C=N Ph
-
Callaho Inc. began operations on January 1 , 2 0 1 8 . Its adjusted trial balance at December 3 1 , 2 0 1 9 and 2 0 2 0 is shown below. Other information regarding Callaho Inc. and its activities...
-
Required: 1. Complete the following: a. Colnpute the unit product cost under absorption costing. b. What is the company's absorption costing net operating income (loss) for the quarter? c. Reconcile...
-
Bond Valuation with Semiannual Payments Renfro Rentals has issued bonds that have an 8% coupon rate, payable semiannually. The bonds mature in 6 years, have a face value of $1,000, and a yield to...

Study smarter with the SolutionInn App


