Benzene and alkyl-substituted benzenes can be hydroxylated by reaction with H2O2 in the presence of an acidic
Question:
Benzene and alkyl-substituted benzenes can be hydroxylated by reaction with H2O2 in the presence of an acidic catalyst. What is the structure of the reactive electrophile? Propose a mechanism for thereaction.
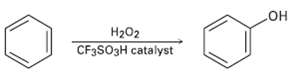
Transcribed Image Text:
H202 CF3SO3H catalyst
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 38% (21 reviews)
HOOH acid catalyst HOOH HOOH reactiv...View the full answer

Answered By

Brown Arianne
Detail-oriented professional tutor with a solid 10 years of experience instilling confidence in high school and college students. Dedicated to empowering all students with constructive feedback and practical test-taking strategies. Effective educator and team player whether working in a school, university, or private provider setting. Active listener committed to helping students overcome academic challenges to reach personal goals.
4.60+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Propose a mechanism for each reaction. (a) (b) (c) (d) OH H2SO heat OCH + CH3OH H20 CH2OH H2So4 heat CH OCH2CH CH CH,CH2OH (a minor product)
-
A carbohydrate (S) decomposes in the presence of an enzyme (E) The Michaelis-Menten kinetic parameters were found to be as follows M 200 mol m 3 100 mol m 3 min a Calculate the change of substrate...
-
Propose a mechanism for each reaction. (a) (b) Ht H3C CH3 OH H SOA Ph Ph Ph Ph
-
Which statements best describe the result of executing this code? (Choose two.) A. The println() causes one line of output. B. The println() causes two lines of output. C. The println() causes three...
-
Wesley and Myrtle (ages 90 and 88, respectively) live in an assisted care facility and for 2015 and 2016 received their support from the following sources:...
-
Zagat restaurant guides publish ratings of restaurants for many large cities around the world (see www.zagat.com). The review for each restaurant gives a verbal summary as well as a 0- to 30-point...
-
Journ Co. purchased short-term investments in available-for-sale securities at a cost of $50,000 on November 25, 2008. At December 31, 2008, these securities had a market value of $47,000. This is...
-
Backflushing. The following conversation occurred between Brian Richardson, plant manager at Glendale Engineering, and Charles Cheng, plant controller. Glendale manufactures automotive component...
-
I need help with the whole question with a solution and explanation Please and Thank you. Chapter 15 Homework Help Save & Exit Submit Check my work At the beginning of a year, a company predicts...
-
A marketing executive tested two incentives to see what percentage of customers would enrol in a new web-based loyalty program. The customers were asked to log on to their accounts on the web and...
-
Treatment of p-tert-butyl phenol with a strong acid such as H2SO4 yields phenol and 2-methyipropene. Propose a mechanism.
-
How would you synthesize the following compounds from benzene? Assume that ortho and Para isomers can beseparated. (b) CH2CHCH3 (a) CH Br O2N SO3H
-
Why do we use quotation marks around the word true in the statement that accuracy refers to how close a measured value is to the true value?
-
Following the example shown in (a) below, indicate the effects of the listed transactions on the assets, liabilities, and stockholders equity of John Dallmus, certified public accountant, a...
-
What effect does the ordering of a search tree have on the efficiency of the search? What effect does it have on the quality of the results? How would order affect the way that depth-first search or...
-
For each of the accounts listed below, indicate whether the account is increased by a debit or a credit: Accounts Receivable Sales Revenue Equipment Common Stock Notes Payable Retained Earnings...
-
Smart Sports is also planning to launch a range of drinks products. The products have been developed by Hydration Labs Ltd and are designed to be sold as powders that dissolve easily in water. They...
-
Baucom Company accepted credit cards in payment for \(\$ 6,850\) of services performed during March 2011. The credit card company charged Baucom a 4 percent service fee. The credit card company paid...
-
Does Starbucks care too much for its partners? Can a company ever treat its employees too well? Why or why not?
-
Synthesize the products by drawing out reagents and intermediates along the way. `N H. OH HO HO
-
As in Illustration 5.1-1 it is desired to produce liquefied methane; however, the conditions are now changed so that the gas is initially available at 1 bar and 200 K, and methane leaving the cooler...
-
Provide names for thesecompounds: CI b) CH;CH,COCH,CH3 a) C-OCH,CH,CH3 cuandoen, c) CH,CH,COCH3 d) CH,CH e) NHCH3 h) CN i)
-
Draw structures for these compounds: (a) Propanoyl chloride (b) N, N-Dimethylacetamide (c) Pentatonic anhydride (d) Sodium p-nitro benzoate (e) Hexanamide (f) Isopropyl acetate (g) Benzyl benzoate...
-
Explain which compound has the higher melting point or boilingpoint: a) Higher mp CH;CH,CH,NH, or CH;CN(CH3)2 b) Higher bp CH,CH,CH,COH or CH,CH,COCH; COH CH c) Higher bp CH, or Higher mp...
-
Cash from Operating Activities: ______________ Cash from Investing Activities: ______________ Cash from Financing Activities: ______________ Problem 2: Financial Ratios The GAP Macys 1 Current Ratio...
-
On January 1, 2021, Winky Enterprises issued 12% bonds dated January 1, 2021, with a face amount of $2,800,000. The bonds mature in 2030 (10 years). For bonds of similar risk and maturity, the market...
-
Using the following accounts and balances, prepare the stockholders' equity vection of the balance sheet. Pilty thousand shares of common stock are authorised, and 1,000 shares have been recoured,...

Study smarter with the SolutionInn App


