How would you carry out the following transformation, a step used in the commercial synthesis of(S)-ibuprofen? CN
Question:
How would you carry out the following transformation, a step used in the commercial synthesis of(S)-ibuprofen?
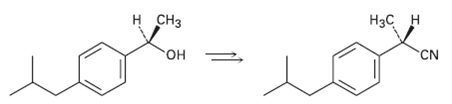
Transcribed Image Text:
н снз Нас н он CN
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (17 reviews)
Strategy Recall from Chapter 11 that OH is a very poor leaving group in re...View the full answer

Answered By

Kalyan M. Ranwa
I have more than seven years of teaching experience in physics and mechanical engineering.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How would you carry out the following synthesis (more than one step is required)? What stereo chemical relationship between the ?CO 2 CH 3 group attached to the cyclohexane ring and the ?CHO groups...
-
How would you carry out the following transformations? More than one step may berequired. H-CH (a) (b) Br - (c) " - " - (d) CH3CH2CH2CH2C=CH CHH2CH2CH2CH2H20CH3 H (e) CH3CH2CH2CH2CHCH3...
-
How would you carry out the following transformations? Co .CO2H (a) (b) Co CH2 C (c) CH2SH
-
Jeremy acquired the following ordinary shares in Scarlon plc: He made no further acquisitions during 2021. On 22 December 2020, he sold 10,000 shares in the company for 10 per share. Calculate the...
-
How do functional tactics differ from corporate and business strategies?
-
Reconsider the previous exercise about sex and coff ee consumption. The sample data could be organized in a two-way table as follows: a. Fill in the table with counts (which you make up) in such a...
-
P6-8 Consolidation workpaper (upstream sales) Financial statements for Pam and Sun Corporations for 2016 are as follows (in thousands): Pam Sun Combined Income and Retained Earnings Statement for the...
-
Alyeski Tours operates day tours of coastal glaciers in Alaska on its tour boat the Blue Glacier. Management has identified two cost driversthe number of cruises and the number of passengersthat it...
-
Non-Tax Oriented Lease: Net Advantage to Leasing Dunbar Corporation can purchase an asset for $30,000; the asset will be worthless after 15 years. Alternatively, it could lease the asset for 15 years...
-
A store maintains data on customers, products and purchase records in three tables: CUSTOMER, PRODUCT, PURCHASE. The store manager wants to know which product is on its maximum discount for each...
-
Use the reaction of a Grignard reagent with a carbonyl compound to synthesize the following compound:
-
What product(s) would you expect from dehydration of the following alcohols with POCl3 in pyridine? Indicate the major product in eachcase? (a) (b) (c) CH3CH2CHCHCH3 CH CH3 H (d) (e) CCCH2CH...
-
A car is moving with speed 20 m/s and acceleration 2 m/s2 at a given instant. Using a second-degree Taylor polynomial, estimate how far the car moves in the next second. Would it be reasonable to use...
-
Carla Vista Corp. sponsors a defined benefit pension plan for its employees. On January 1, 2025, the following balances relate to this plan Plan assets $489,900 Projected benefit obligation 616,700...
-
Question 2 of 8 Shirts were purchased for $12.50 each and were marked up by $18.75. During Christmas, they were discounted by $6.85 per shirt. a. What was the rate of markdown? % Round to two decimal...
-
The cost versus quality decision is one that only few companies get right. What is the cost of quality? It is very high for some companies such as Ford and Bridgestone/Firestone, whose reputations...
-
Find the absolute maximum and absolute minimum values of the function f(x) (x-2)(x-5)+7 = on each of the indicated intervals. Enter 'NONE' for any absolute extrema that does not exist. (A) Interval =...
-
4. Roll one 10-sided die 12 times. The probability of getting exactly 4 eights in those 12 rolls is given by (a) 10 9 4 10 10 (b) HA 9 -HAA (c) 1 (d) 9 (c) 10 9 () 10
-
Zheng Chung is the managing owner of the Stillwater Grill. This year, 2010, was a good year for him. Now he is preparing his 2011 budget. Using the information Zheng believes will be true, help him...
-
Which should drive action planning more, strengths or weaknesses? That is, is it more important to build on your strengths or to reduce your weaknesses? Explain.
-
Would you expect an ionic solid or a network covalent solid to have the higher melting point?
-
First, complete and balance each of the equations below. Then, choosing among ethanol, hexane, and liquid ammonia, state which (there may be more than one) might be suitable solvents for each of...
-
Dimethylformamide (DMF), HCON(CH3)2, is an example of a polar aprotic solvent, aprotic meaning it has no hydrogen atoms attached to highly electronegative atoms. (a) Draw its dash structural formula,...
-
As noted in Table 3.1, the pKa of acetone, CH3COCH3, is 19.2. In Table 3.1 (a) Draw the bond-line formula of acetone and of any other contributing resonance form. (b) Predict and draw the structure...
-
The payroll register of Ruggerio Co. indicates $13,800 of social security withheld and $3,450 of Medicare tax withheld on total salaries of $230,000 for the period. Federal withholding for the period...
-
All of the following are included on Form 1040, page 1, EXCEPT: The determination of filing status. The Presidential Election Campaign check box. The income section. The paid preparer signature line.
-
Question One: (25 marks) (X) Inc. purchased 80% of the outstanding voting shares of (Y) for $360,000 on July 1, 2017. On that date, (Y) had common shares and retained earnings worth $180,000 and...

Study smarter with the SolutionInn App


