How would you carry out the following synthesis (more than one step is required)? What stereo chemical
Question:
How would you carry out the following synthesis (more than one step is required)? What stereo chemical relationship between the ?CO2CH3 group attached to the cyclohexane ring and the ?CHO groups would your synthesis produce?
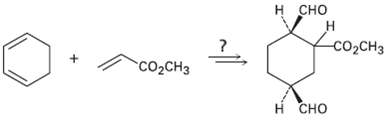
Transcribed Image Text:
н сно H. со,CHз "сооснз н сно
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 64% (14 reviews)
Although it is usually best to work backwards in a synthesis ...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How would you carry out the following transformations? Co .CO2H (a) (b) Co CH2 C (c) CH2SH
-
How would you carry out the following transformations? "Co CH2 C Lc
-
How would you carry out the followingreactions? (a) CCH-CCH3 CH3CH2C=CH () H2C%3CH CH3CH2CH2CHO .3- (c) 3C (d) CH . CH 7, 22H (e) CH3CH2C=CH (f) CH3CH2CH2CH2CH=CH2 CHCH2CH2CH2C%CH (2 steps)
-
in an armicie entitled Fuel Economy Calculations tio Be Alteted lames Healey indicated that the goermnt planned to change how it caleulates fuel economy for new cas and trucks Thia in the first...
-
How could the services firm in the second example have met the requirements for overtime pay?
-
Let Compute the indicated matrices (if possible). F(AF) 2 1 C = 3 |A = 4 B = -2 3 4 2 3 5 5 6 -3 E = [4 2], F= D = %3D 2.
-
How should Tom assess the current situation? LO.1
-
During 2017 the Lung Association received a contribution of marketable securities that were to be placed in a permanent endowment fund. Neither donor stipulations nor applicable state law requires...
-
Fanning Corporation operates three investment centers. The following financial statements apply to the investment center named Bowman Division. BOWMAN DIVISION Income Statement For the Year Ended...
-
As the promotional manager for a new line of cosmetics targeted to preteen girls, you have been assigned the task of deciding which promotional mix elements - advertising, public relations, sales...
-
Dimethyl butynedioate also undergoes a DielsAlder reaction with (2E, 4Z)-hexadiene, but the stereochemistry of the product is different from that of the (2E, 4E) isomer (Problem 14.55). Explain.
-
The double bond of an enamine (alkene amine) is much more nucleophilic than a typical alkene double bond. Assuming that the nitrogen atom in an enamine is sp2-hybridized, draw an orbital picture of...
-
What are the key elements of the commitment strategy from the universalistic approach? Why does this approach generally contribute to success for organizations?
-
The transmitted energy expands out into space as it propagates at 3 GHz between the transmitter and the receiver over 30 km distance. Calculate the free space loss using a suitable formula and any...
-
What is the company featured in this episode of Undercover Boss? List 3 good professional activities that the CEO/president learned about their company by going undercover? List areas of the...
-
Assume there is a national lottery in the winning ticket is worth $10 million one winning ticket will be selected if there are 225 million tickets sold. What is the chance that a buyer of one ticket...
-
Description: Reference: Basu Thakur. (2015). PostcolonialTheory and Avatar (pp. 85-150,157-172). Bloomsbury PublishingUSAPre-Peer Paper Review for the Postcolonial Application Paper 1: Collecting...
-
NOT ASKING THE ACTUAL SHEAR STRESS. Please READ! Derive the shear stress distributed equation over the cross-section. Derive the equation and plot. 15 15 30 15 15 120 -90 20 0.5 m 72 kN 20 20 40 40...
-
Find an academic research report. Just about any article from Academy of Management Review, Journal of Management, Journal of Marketing, Journal of Business Research, or Journal of Retailing, among...
-
You are a Loan Officer with an Investment Bank. Today you need to set your lending parameters. They are: LTV: 55% 10 Year T-Bill: TBD Rate Markup: 300 Basis Points Term: 30 Years Amortization: 30...
-
What volume (in mL) of a soft drink that is 10.5% sucrose (C 12 H 22 O 11 ) by mass contains 78.5 g of sucrose? (The density of the solution is 1.04 g/mL.) SORT You are given a mass of sucrose and...
-
Predict the major products formed when the following amines undergo exhaustive methylation, treatment with Ag2O, and heating. (a) Hexan-2-amine (b) 2-methylpiperidine (c) N-ethylpiperidine (d) (e)...
-
Give the products expected when the following tertiary amines are treated with a peroxyacid and heated. (a) N, N-dimethylhexan-2-amine (b) N, N-diethylhexan-2-amine (c) Cyclohexyldimethylamine (d)...
-
When the (R, R) isomer of the amine shown is treated with an excess of methyl iodide, then silver oxide, then heated, the major product is the Hofmann product. (a) Draw the structure of the major...
-
Practice Problem 1 The stockholders equity accounts of Bramble Corp. on January 1, 2017, were as follows. Preferred Stock (6%, $100 par noncumulative, 4,400 shares authorized) $264,000 Common Stock...
-
JVCU Which of the following is considered cash for financial reporting purposes? 1 JVCU Which of the following is considered cash for financial reporting purposes? 1
-
Required information The Foundational 15 [LO8-2, LO8-3, LO8-4, LO8-5, LO8-7, LO8-9, L08-10) (The following information applies to the questions displayed below.) Morganton Company makes one product...

Study smarter with the SolutionInn App


