How would you carry out the followingreactions? (a) CCH-CCH3 CH3CH2C=CH () H2C%3CH CH3CH2CH2CHO .3- (c) 3C (d)
Question:
How would you carry out the followingreactions?
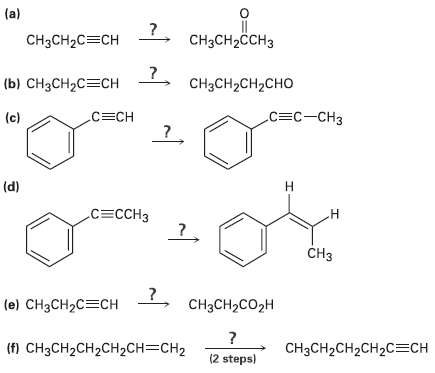
Transcribed Image Text:
(a) CнзCH-CCH3 CH3CH2C=CH (ы) снзсH2C%3CH CH3CH2CH2CHO .С3с-сНз (c) С3CН (d) н СЕСCHЗ .н CHз 7, снасн2со2H (e) CH3CH2C=CH (f) CH3CH2CH2CH2CH=CH2 CHзCH2CH2CH2C%ЕCH (2 steps)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (15 reviews)
b c CH3CHCCH CH3CHCCH CCH HO HSO4 HgSO4 1 BH3 THF ...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How would you carry out the following transformations? More than one step may berequired. H-CH (a) (b) Br - (c) " - " - (d) CH3CH2CH2CH2C=CH CHH2CH2CH2CH2H20CH3 H (e) CH3CH2CH2CH2CHCH3...
-
How would you carry out the following transformation, a step used in the commercial synthesis of(S)-ibuprofen? CN
-
How would you carry out the following transformations? Co .CO2H (a) (b) Co CH2 C (c) CH2SH
-
At December 31, 2014, Torrealba Company reported the following as plant assets. During 2015, the following selected cash transactions occurred. April 1 Purchased land for $1,200,000. May 1 Sold...
-
A set of ten jobs must be scheduled at an integrated machining center that performs a number of metal-cutting operations on components for complex assemblies. These jobs and their processing times...
-
An experimental fluid is used to create a spherical bubble with a diameter of 0.25 cm. When in contact with a surface made of plastic, it has a contact angle of 30 degrees. The pressure inside the...
-
Project Cost Management a. Assume that you have three people working on the project and each of them would charge $20 per hour. Enter this information in the Resource Sheet. b. Estimate that each...
-
Juarez Corporation produces cleaning compounds and solutions for industrial and household use. While most of its products are processed independently, a few are related. Grit 337, a coarse cleaning...
-
The final answer is: Here's the final formula: =SWITCH(A1>=3.5,A,A1>=3.0,B,A1>=2.5,C,A1>=2.0,D,TRUE,F) Replace A1 with the actual cell reference where the student's GPA is located in your Excel sheet
-
Determine whether or not F is a conservative vector field. If it is, find a function f such that F = f. F(x, y) = ye x i + (e x + e y ) j
-
Hydrocarbon A has the formula C 9 H 12 and absorbs 3 equivalents of H 2 to yield R, C 9 H 18 , when hydrogenated over a Pd/C catalyst. On treatment of A with aqueous H 2 SO 4 in the presence of...
-
Occasionally, chemists need to invert the stereochemistry of an alkene?that is, to convert a cis alkene to a trans alkene, or vice versa. There is no one-step method for doing an alkene inversion,...
-
Describe how you will remember the forward- and reverse-bias states of the p n junction diode. That is, how will you remember which potential (positive or negative) is applied to which terminal?
-
Low Desert Pottery works makes a variety of pottery products that it sells to retailers. The company uses a job-order costing system in which departmental predetermined overhead rates are used to...
-
ASSESSMENT CPCCBC5002A Monitor costing systems on medium rise building and construction projects Please provide answer to Part 2 - Monitor expenditure for a medium-rise project as per below...
-
Questions 6-8 refer to the same problem A sinusoidal wave with wavelength 2 m and amplitude 5 mm is traveling along the x axis. The wave is traveling in the -x direction at a speed of 2m/s At t = Os,...
-
Consider a circuit where one or more capacitors is discharged through a light bulb filament with a resistance of 3.0 0.3 . Assume that the resistance of the filament is constant (to within the stated...
-
3. For a vibrating string of length with fixed ends, each mode of vibration can be written as where wk ux(x, t) = M* sin(wxt + k) sin(x) and Mk, Ok are determined by initial conditions. For all k >...
-
Organization: Catalyst Event Solutions Web site: www.catalystevents .com.au Summary: Catalyst Event Solutions is a conference and event management company in Sydney that offers both single services,...
-
The May 2014 revenue and cost information for Houston Outfitters, Inc. follow: Sales Revenue (at standard).............. $ 540,000 Cost of Goods Sold (at standard) ..........341,000 Direct Materials...
-
Consider the precipitation reaction: What volume of 0.175 M Na3PO4 solution is necessary to completely react with 95.4 mL of 0.102 M CuCl 2 ? 2 Na3PO4(aq) + 3 CuCl(aq) Cu3(PO4)2 (s) + 6 NaCl(aq)
-
Draw a Lewis structure for each species. (a) N2H4 (b) N2H2 (c) (CH3)2NH2CI (d) CH3CN (e) CH3CHO (f) CH3S(O)CH3 (g) H2SO4 (h) CH3NCO (i) CH3OSO2OCH3 (j) CH3C(NH)CH3 (k) (CH3)3CNO
-
Draw a Lewis structure for each compound. Include all nonbonding pairs of electrons. (a) CH3COCH2CHCHCOOH (b) NCCH2COCH2CHO (c) CH2CHCH(OH)CH2CO2H (d) CH2CHC(CH3)CHCOOCH3
-
Draw a line-angle formula for each compound in Problem 1-26. In problem (a) CH3COCH2CHCHCOOH (b) NCCH2COCH2CHO (c) CH2CHCH(OH)CH2CO2H (d) CH2CHC(CH3)CHCOOCH3
-
Which of the following accounts will not be closed during the closing process? a. Accounts Recelvable b. Wages Expense c. Fees Earned d. Rent Expense
-
Clarkson Lumber Company After a rapid growth in its business during recent years, the Clarkson Lumber Company, in the spring of 1996, anticipated a further substantial increase in sales. Despite good...
-
How do external factors such as changing consumer preferences affect the retail industry?"

Study smarter with the SolutionInn App


