Hydrocarbon A has the formula C 9 H 12 and absorbs 3 equivalents of H 2 to
Question:
Hydrocarbon A has the formula C9H12 and absorbs 3 equivalents of H2 to yield R, C9H18, when hydrogenated over a Pd/C catalyst. On treatment of A with aqueous H2SO4 in the presence of mercury (II), two isomeric ketones, C and D, are produced. Oxidation of A with KMnO4 gives a mixture of acetic acid (CH3CO2H) and the tricarboxylic acid E. Propose structures for compounds A?D, and write the reactions.
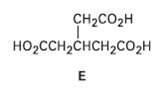
Transcribed Image Text:
CH2CO2H но-сCHснсH2Cо2н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (14 reviews)
CHCHCH CH3 B A HOCCH ...View the full answer

Answered By

Michael Owens
I am a competent Software Engineer with sufficient experience in web applications development using the following programming languages:-
HTML5, CSS3, PHP, JAVASCRIPT, TYPESCRIPT AND SQL.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Propose structures for compounds G-I: concd HNO heat 60-65 OH
-
Hydrocarbon A has the formula C12H8. It absorbs 8 equivalents of H2 on catalytic reduction over a palladium catalyst. On ozonolysis, only two products are formed: oxalic acid (HO2CCO2H) and succinic...
-
Propose structures for compounds E and F. Compound E (C8H6) reacts with 2 molar equivalents of bromine to form F (C8H6Br4). E has the IR spectrum shown in Fig. 9.50. What are the structures of E and...
-
Peabody Coal acquired the mineral rights to a tract of land containing coal deposits. Total costs of acquisition, exploration, development were $1,200,000 and an ARO of $300,000 was recorded. The...
-
During the next 8 months, Metropolis Power Company forecasts the demands shown in the table below (measured in thousands of kwh): Power will be supplied from the four generating facilities, GF1- GF4....
-
Determine the diameter of the glass tube necessary to keep the capillary-height change of water at 30C less than 1 mm.
-
Project Time Management a. Enter realistic durations for each task, and then link the tasks as appropriate. Be sure that all tasks are linked (in some fashion) to the start and end of the project....
-
Part 1 Modify your program from Learning Journal Unit 7 to read dictionary items from a file and write the inverted dictionary to a file. You will need to decide on the following: How to format each...
-
2. Information: Prepaid insurance Accrued salaries Petty cash Investment in trading securities Total current assets Cash Inventory, at sales value (cost= $1,500) Unearned rent Allowance for bad debts...
-
Three 100 ? Resistors are connected as shown in the Figure. The maximum power that can safely be delivered to any one resistor is 22.5 W. (a) What is the maximum potential difference that can be...
-
How would you carry out the following conversions? More than one step may be needed is some instances. CI RCH RCHCH3 RCH2CH3 RCH2CH2OH R-C=CH R-C C-CH3 RCH=CH2 R-C- RCCH3
-
How would you carry out the followingreactions? (a) CCH-CCH3 CH3CH2C=CH () H2C%3CH CH3CH2CH2CHO .3- (c) 3C (d) CH . CH 7, 22H (e) CH3CH2C=CH (f) CH3CH2CH2CH2CH=CH2 CHCH2CH2CH2C%CH (2 steps)
-
You will need to understand solved problem S4 before answering this question .A gene that is normally expressed in pancreatic cell was cloned and then subjected to promoter bashing. As shown here,...
-
Problem 8-19 (Algo) Cash Budget; Income Statement; Balance Sheet [LO8-2, LO8-4, LO8-8, LO8-9, LO8- 10] Minden Company is a wholesale distributor of premium European chocolates. The company's balance...
-
Consider the unsteady flow of a fluid in the x direction through a control volume. The linear momentum of the fluid within the control volume is a function of time given by 200ti slug*ft/s, where t...
-
For a continuous uniform distribution with u = 0 and o = 1, the minimum is - V3 and the maximum is V3. For this continuous uniform distribution, find the probability of randomly selecting a value...
-
Marc Goudreau, administrator of Clearwater Hospital, was puzzled by the prior month's reports. "Every month, it's anyone's guess whether the lab will show a profit or a loss. Perhaps the only answer...
-
A system consisting of a gas consisting of O2 (32 Da), H2 (2 Da), and Ar (40 Da) molecules and a billiard ball is at some temperature . Relative to O2, the billiard ball is 1.0 E+26 times as massive...
-
Organization: Wizcraft Web site: http://www.wizcraftworld .com/ Summary: Wizcraft is an Indiabased communication and entertainment company that caters to a global audience. It has a client base that...
-
What impact has the Internet had on the globalization of small firms? How do you think small companies will use the Internet for business in the future?
-
To what volume should you dilute 25 mL of a 10.0 M H 2 SO 4 solution to obtain a 0.150 M H 2 SO 4 solution?
-
There is a small portion of the periodic table that you must know to do organic chemistry. Construct this part from memory, using the following steps. (a) From memory, make a list of the elements in...
-
For each compound, state whether its bonding is covalent, ionic, or a mixture of covalent and ionic. (a) NaCl (b) NaOH (c) CH3Li (d) CH2CI2 (e) NaOCH3 (f) HCO2Na (g) CF4
-
(a) Both PCI3 and PCI5 are stable compounds. Draw Lewis structures for these two compounds. (b) NCI3 is a known compound, but all attempts to synthesize NCI5 have failed. Draw Lewis structures for...
-
September 1 . Purchased a new truck for $ 8 3 , 0 0 0 , paying cash. September 4 . Sold the truck purchased January 9 , Year 2 , for $ 5 3 , 6 0 0 . ( Record depreciation to date for Year 3 for the...
-
Find the NPV for the following project if the firm's WACC is 8%. Make sure to include the negative in your answer if you calculate a negative. it DOES matter for NPV answers
-
What is the value of a 10-year, $1,000 par value bond with a 12% annual coupon if its required return is 11%?

Study smarter with the SolutionInn App


