Occasionally, chemists need to invert the stereochemistry of an alkene?that is, to convert a cis alkene to
Question:
Occasionally, chemists need to invert the stereochemistry of an alkene?that is, to convert a cis alkene to a trans alkene, or vice versa. There is no one-step method for doing an alkene inversion, but the transformation can be carried out by combining several reactions in the proper sequence. How would you carry out the following reactions?
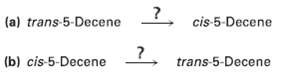
Transcribed Image Text:
5 cis-5-Decene (a) trans 5-Decene (b) cis-5-Decene trans-5-Decene
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (11 reviews)
a b trans5Decene cis5Decene ...View the full answer

Answered By

Rohail Amjad
Experienced Finance Guru have a full grip on various sectors, i.e Media, Insurance, Automobile, Rice and other Financial Services.
Have also served in Business Development Department as a Data Anlayst
4.70+
32+ Reviews
83+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The production of 1,3-butadiene can be carried out by the dehydrogenation of 1- butene: C2H5CH:CH2(g) CH2:CHCH:CH2(g) + H2(g) Side reactions are suppressed by the introduction of steam. If...
-
The production of 1,3-butadiene can be carried out by the dehydrogenation of n- butane: C4H 10(g) CH2 : CHCH : CH2(g) + 2H2(g) Side reactions are suppressed by the introduction of steam. If...
-
How would you carry out the following reactions to introduce deuterium into organicmolecules? (a) CHH2C3CCH2C Hs 2H5 (b) C2H5 CHH2C3CCH2CH3 C=C Hs D (c) CH3CH2CH2C=CH CH3CH2CH2C=CD CD CD2 C=CH (d)
-
Super Slushie charges $6.50 for a medium slushie and $9 for a large slushie. Their total net marketing contribution is $28,000 per week. They want to raise the price of each slushie 12.5% next month...
-
You are organizing rides for a group of campers going on an all-day, off-site trip. You have lined up some drivers, and your problem is to assign campers to drivers. The drivers and the capacities of...
-
A colleague is trying to measure the diameter of a capillary tube, something that is very difficult to physically accomplish. Since you are a Fluid Dynamics student, you know that the diameter can be...
-
Project Human Resource Management a. Assume that one project team member will be unavailable (due to vacation) for two weeks in the middle of the project. Make adjustments to accommodate this...
-
Tiny Town Kennel earns service revenue by caring for the pets of customers. Tiny Town Kennel is organized as a corporation. During the past month, Tiny Town Kennel has the following transactions: a....
-
A manufacturer can produce an item for $141/unit with a fixed cost of $31,826. The alternative is to outsource the production to a supplier at a unit cost of $224/unit. Determine the BEP $...
-
Evaluate the sales promotion strategies used by Tim Hortons. What marketing and marketing communications objectives do they meet? How does Tim Hortons integrate sales promotions with other components...
-
How would you carry out the followingreactions? (a) CCH-CCH3 CH3CH2C=CH () H2C%3CH CH3CH2CH2CHO .3- (c) 3C (d) CH . CH 7, 22H (e) CH3CH2C=CH (f) CH3CH2CH2CH2CH=CH2 CHCH2CH2CH2C%CH (2 steps)
-
Propose structures for hydrocarbons that give the following products on oxidative cleavage by KMnO4 orO3: (b) .Co2 (a) O2 + CH(CH2)5C02H CC2H + e) O2CICH2)8CO2H (d) CH + 2H2cO2H + co2 (e) CO2...
-
You need to perform gravimetric analysis of a water sample in order to determine the amount of Ag + present. a. List three aqueous solutions that would be suitable for mixing with the sample to...
-
The combined weight of the load and the platform is 200 lb, with the center of gravity located at G. If a couple moment of M = 900 lb ft is applied to link AB, determine the angular velocity of links...
-
Due In: 06:48:23 Questions Question 1 (4) O Question 2 (8) Question 2 of 2 A company sold $150,000 bonds and set up a sinking fund that was earning 8.5% compounded semi-annually to retire the bonds...
-
Find the point on the graph of f(x) = x which is closest to the point (6, 27). How close is the closest point?
-
Due to a crash at a railroad crossing, an overpass is to be constructed on an existing level highway. the existing highway has a design speed of 50 mi/h. The overpass structure is to be level,...
-
Finding Bone Density Scores. In Exercises 37-40 assume that a randomly selected subject is given a bone density test. Bone density test scores are normally distributed with a mean of 0 and a standard...
-
Make a master plan that includes the necessary steps to hold a meeting or seminar on careers in hospitality management. LO.1
-
One Way Cellular accountants have assembled the following data for the year ended September 30, 2014: Prepare the operating activities section using the indirect method for One Way Cellulars...
-
To what volume should you dilute 50.0 mL of a 12 M stock HNO 3 solution to obtain a 0.100 M HNO 3 solution?
-
Draw Lewis structures for (a) Two compounds of formula C4H10 (b) Two compounds of formula C2H6O (c) Two compounds of formula C2H7N (d) Three compounds of formula C2H7NO (e) Three compounds of formula...
-
Draw a complete structural formula and a condensed structural formula for (a) Three compounds of formula C3H8O (b) Five compounds of formula C3H6O
-
Write Lewis structures for the following molecular formulas. (a) N2 (b) HCN (c) HONO (d) CO2 (e) CH3CHNH (f) HCO2H (g) C2H3CI (h) HNNH (i) C3H6 (one double bond) (j) C3H4 (two double bonds) (k) C3H4...
-
Deacon Company is a merchandising company that is preparing a budget for the three - month period ended June 3 0 th . The following information is available Deacon Company Balance Sheet March 3 1...
-
Mango Company applies overhead based on direct labor costs. For the current year, Mango Company estimated total overhead costs to be $460,000, and direct labor costs to be $230,000. Actual overhead...
-
Which of the following do we expect to be the horizon growth rate for a company (long term growth rate- say 30-50 years)? A) Inflation B) Industry Average C) Zero D) Market Beta

Study smarter with the SolutionInn App


