Question: Allowed values for the quantum numbers of electrons are as follows: The relationships between n and the shell designations are noted in Table 2.1. Relative
Allowed values for the quantum numbers of electrons are as follows:
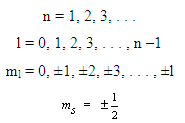
The relationships between n and the shell designations are noted in Table 2.1. Relative to the subshells,
l = 0 corresponds to an s subshell
l = 1 corresponds to a p subshell
l = 2 corresponds to a d subshell
l = 3 corresponds to an f subshell
For the K shell, the four quantum numbers for each of the two electrons in the 1s state, in the order of nlmlms, are 100() and 100(). Write the four quantum numbers for all of the electrons in the L and M shells, and note which correspond to the s, p, and d subshells.
n = 1, 2, 3, ... 1= 0, 1, 2, 3, ... , n-1 m = 0, =1, 2, +3, ..., 1 +I
Step by Step Solution
3.40 Rating (162 Votes )
There are 3 Steps involved in it
Representation of L state is given as n 2 and 8 electro... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (6).docx
120 KBs Word File


