Amine bases in nucleic acids can react with alkylating agents in typical SN2 reactions. Look at the
Question:
Amine bases in nucleic acids can react with alkylating agents in typical SN2 reactions. Look at the following electrostatic potential maps, and tell which is the better nucleophile, guanine or adenine. The reactive positions in each areindicated.
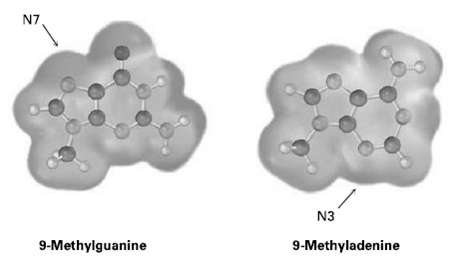
Transcribed Image Text:
N7 N3 9-Methyladenine 9-Methylguanine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
According to the electrostatic potential map the ...View the full answer

Answered By

Somshukla Chakraborty
I have a teaching experience of more than 4 years by now in diverse subjects like History,Geography,Political Science,Sociology,Business Enterprise,Economics,Environmental Management etc.I teach students from classes 9-12 and undergraduate students.I boards I handle are IB,IGCSE, state boards,ICSE, CBSE.I am passionate about teaching.Full satisfaction of the students is my main goal.
I have completed my graduation and master's in history from Jadavpur University Kolkata,India in 2012 and I have completed my B.Ed from the same University in 2013. I have taught in a reputed school of Kolkata (subjects-History,Geography,Civics,Political Science) from 2014-2016.I worked as a guest lecturer of history in a college of Kolkata for 2 years teaching students of 1st ,2nd and 3rd year. I taught Ancient and Modern Indian history there.I have taught in another school in Mohali,Punjab teaching students from classes 9-12.Presently I am working as an online tutor with concept tutors,Bangalore,India(Carve Niche Pvt.Ltd.) for the last 1year and also have been appointed as an online history tutor by Course Hero(California,U.S) and Vidyalai.com(Chennai,India).
4.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Look at the following electrostatic potential map of chloromethane, and tell the direction of polarization of the C ? C1 bonds: CI C-H Chloromethane H.
-
Which is a better nucleophile in methanol? a. H2O or HO- b. NH3 -NH2 c. H2O or H2S d. HO- or HS- e. I- or Br - f. Cl- Br-
-
The following electrostatic potential diagrams represent H2, HCl, or NaCl. Label each, and explain your choices. (a) (b)
-
Sam's Furniture uses variance analysis to evaluate manufacturing overhead in its' factory. The information for the June overhead expenditures is as follows: Budgeted output units 22,000...
-
What four factors are determinants of national advantage and serve as a basis for international business-level strategies?
-
Physicists first attempted to understand the hydrogen atom by applying the laws of classical physics. Consider an electron of mass m and charge -e in a circular orbit of radius r around a proton of...
-
What is necessary for disaster preparedness? LO.1
-
South Atlantic Chemical Company manufactures industrial chemicals in Rio de Janeiro, Brazil. The company plans to introduce a new chemical solution and needs to develop a standard product cost. The...
-
Not yet answered Points out of 1.00 P Has question Snaxx Corp. produces jumbo-sized bags of hot fries snacks. Its current fixed cost is $40,000. The variable costs are $60 per bag. With a production...
-
Brothers Herm and Steve Hargenrater began operations of their tool and die shop (H & H Tool) on January 1, 1987, in Meadville, PA. The annual reporting period ends December 31. Assume that the trial...
-
Identify the following nucleotide, and tell how it is used:
-
Human brain natriuretic peptide (BNP) is a small peptide of 32 amino acids used in the treatment of congestive heat failure. How many nitrogen bases are present in the DNA that codes for BNP?
-
Draw a short segment of gutta-percha.
-
What is the average age (measured by the variable "age") of the sample in the GSS93 subset.sav data set? Is there a significant difference in the age of those who favor the death penalty for murder...
-
Solve the system of linear equations, using the Gauss-Jordan elimination method. (If there is no solution, enter NO SOLUTION. If there are infinitely many solutions, express your answer in terms of...
-
The pay disparity is due to several reasons, one of the main ones being the old stereotypes based on the archetype of the man as the breadwinner of the family. Women are usually hired at a lower...
-
Prepare Balance Sheet: To do this activity you are required to assume the amount and line items that are to be shown on the balance sheet of your business selling homemade articles. Using the...
-
You have a "Consent to Use E-mail Communication" on file for this patient. Draft a short e-mail to her about her lab and chest X-ray results, requesting she contact the office by phone or e-mail to...
-
Thinking that short-term goals are always higher in priority than long-term goals. LO.1
-
The test statistic in the NeymanPearson Lemma and the likelihood ratio test statistic K are intimately related. Consider testing H 0 : = 0 versus H a : = a , and let * denote the test statistic...
-
Consider this three-step mechanism for a reaction: a. What is the overall reaction? b. Identify the intermediates in the mechanism. c. What is the predicted rate law? k Cl(8) k Cl(g) + CHCl3(8)...
-
Write the structure of the product expected from the reaction of alanylcysteine with a mild oxidizing agent, such as hydrogen peroxide For Information: (see Sec. 17.8).
-
Write equations for the following reactions of Sanger's reagent: a. 2, 4-dinitrofluorobenzene + glycine b. Excess 2, 4-dinitrofluorobenzene + lysine
-
Examine the structure of cyclosporin A (page 503). a. By drawing a dashed line at each peptide bond, deduce how many amino acid units are present in cyclosporin A. b. Three of the units are...
-
Be prepared to explain the texts comprehensive To illustrate the issues related to interest capitalization, assume that on November 1, 2016, Shalla Company contracted Pfeifer Construction Co. to...
-
On April 1, 2020. Indigo Company received a condemnation award of $473,000 cash as compensation for the forced sale of the company's land and building, which stood in the path of a new state highway....
-
The market price of a stock is $24.55 and it is expected to pay a dividend of $1.44 next year. The required rate of return is 11.23%. What is the expected growth rate of the dividend? Submit Answer...

Study smarter with the SolutionInn App


