Amino acids contain both a basic functional group, the amine, and an acidic functional group, the carboxylic
Question:
Amino acids contain both a basic functional group, the amine, and an acidic functional group, the carboxylic acid. Thus, they can undergo an internal acid-base re-action as shown in the following equation for the amino acid phenylalanine:
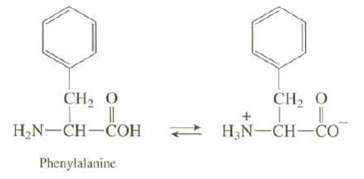
Using Table 4.2 and neglecting the effect of one group on the acidity of the other, predict the position of this equilibrium in the case of phenylalanine. Explain whether your prediction is in accord with the experimental observations that phenylalanine melts at 273-276oC and is very soluble in water.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





