Di-peptides result from the reaction of two amino acids to form an amide. Explain which nitrogen of
Question:
Di-peptides result from the reaction of two amino acids to form an amide. Explain which nitrogen of the following di-peptide is the stronger base:
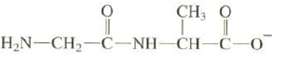
Transcribed Image Text:
CH,O LI H_N–CH, C-NH-CH-C-0
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (18 reviews)
The N that is bonded to the C O group is not very basic beca...View the full answer

Answered By

Chiranjib Thakur
I have no tutoring experience yet, but I can share my skills and knowledge gained from my education and work experiences. I have been a CPA since 2012 with 6 years of work experience in internal auditing and 4 years of work experience in accounting at the supervisory level.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Two substitution products result from the reaction of 3-chloro-3-methyl-1-butene with sodium acetate (CH3COO-Na+) in acetic acid under SN1 conditions. Identify the products
-
Two products are obtained from the reaction of (Z)-2-pentene with water and a trace of H2SO4. The mass spectra of these products are shown in Figure 13.10. Identify the compounds responsible for the...
-
Two stereoisomers are obtained from the reaction of HBr with (S)-4-bromo-1-pentene. One of the stereoisomers is optically active, and the other is not. Give the structures of the stereoisomers,...
-
Recent trends in recruiting rely on social capital by ______. Multiple choice question. making use of structural holes between groups in a social network where there are few relationships bridging...
-
What is the meaning of the term interaction between variances?
-
Pel, a U.S. firm, paid $308,000 for all the common stock of Sar of Israel on January 1, 2016, when the exchange rate for sheqels was $0.35. Sar's equity on this date consisted of 500,000 sheqels...
-
PR 5-2 Does noncontrolling interest represent a liability or an equity in the consolidated balance sheet?
-
In August 2011, the PCAOB barred two former Ernst & Young LLP (E&Y) employees from auditing public companies, alleging they provided misleading documents to PCAOB inspectors who were evaluating the...
-
One bank offers you an APR of 10% interest compounded monthly. What would the equivalent rate be if interest were compounded semiannually? (if you round some of your intermediate results, please keep...
-
Job costing, normal and actual costing. Amesbury Construction assembles residential houses. It uses a job-costing system with two direct-cost categories (direct materials and direct labor) and one...
-
Amino acids contain both a basic functional group, the amine, and an acidic functional group, the carboxylic acid. Thus, they can undergo an internal acid-base re-action as shown in the following...
-
Explain which nitrogen in the ring of the amino acid histidine is the stronger base: N- N-H CH + HN CHCO, Histidine
-
Mystic Pizza Company purchased a patent from Prime Pizza Plus on January 1, 2019, for $72,000. The patent has a remaining legal life of 9 years. Prepare the journal entries to record the acquisition...
-
a ) If F ( x ) = upper limit x and lower limit 8 dt , the F \' ( x ) = ? b ) If F ( x ) = upper limit 1 1 and lower limit x dt , the F \' ( x ) = ? c ) If F ( x ) = upper limit x ^ 6 and lower limit...
-
Please help me with this one can you do for me 1. the research on quantum dots. Find a resource that you think does the best job of explaining this. and provide short description of what you've...
-
During Year 1, Ashkar Company ordered a machine on January 1 at an invoice price of $28,000. On the date of delivery, January 2, the company paid $9,000 on the machine, with the balance on credit at...
-
An airplane has to travel a round trip Manila to Singapore and back (1485 mi apart) in a total time of 7 hours and 10 minutes. The plane from manila to Singapore is flying with a headwind of 70 mph...
-
Flexible Budget. At normal capacity, Boulder Products Corp. manufactures 10,000 trail bikes. At that level, unit variable costs for the Assembly Department are: Direct...
-
Explain the usefulness of knowing the energy costs.
-
Define a traverse in Surveying?
-
Sketch the graph of the equation. y = 2
-
The pKa of p-cyclopropylbenzoic acid is 4.45. Is cyclopropylbenzene likely to be more reactive or less reactive than benzene toward electrophilic bromination? Explain.
-
Rank the following compounds in order of increasing acidity. Dont look at a table Of PKa data to help with your answer. (a) Benzoic acid, p-methyl benzoic acid, p-chlorohenzoic acid (b)...
-
How would you prepare the following carboxylic acids? (a) (CH 3 ) 3 CCO 2 H from (CH 3 ) 3 CCl (b) CH 3 CH 2 CH 2 CO2H from CH 3 CH 2 CH 2 Br
-
Your firm is planning to invest in an automated packaging plant. Harburtin Industries is an all - equity firm that specializes in this business. Suppose Harburtin ' s equity beta is 0 . 8 7 , the...
-
Ned Allen opened a medical practice in Los Angeles, California, and had the following transactions during the month of January. (Click the icon to view the January transactions.) Journalize the...
-
do you need more information or are you working on this? Irene Watts and John Lyon are forming a partnership to which Watts will devote one- half time and Lyon will devote full time. They have...

Study smarter with the SolutionInn App


