Explain which nitrogen in the ring of the amino acid histidine is the stronger base: N- N-H
Question:
Explain which nitrogen in the ring of the amino acid histidine is the stronger base:
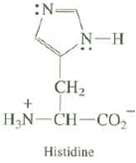
Transcribed Image Text:
N- N-H CH₂ + HẠN CH–CO, Histidine
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (14 reviews)
The electrons on the N with the H are part of the ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Histidine is an amino acid with three titratable groups: an - NH3+ group (pKa = 9.2), a -COOH group 1 (pKa = 1.8), and an imidazole (amine-like) group 1 (pKa = 6.0). Te titration curve for histidine...
-
The codon CAC corresponds to the amino acid histidine (His). How will this codon appear in the DNA strand from which it was transcribed? In the complement of that strand? Be sure to label the 5' and...
-
Explain the following observations: (a) HCl is a stronger acid than H2S; (b) H3PO4 is a stronger acid than H3AsO4; (c) HBrO3 is a stronger acid than HBrO2; (d) H2C2O4 is a stronger acid than HC2O4-;...
-
The need to be liked and to stay on good terms with most other people is the need for? a. Affiliation b. Power c. None of the above d. Achievement
-
Explain why a favourable variance does not necessarily indicate good performance?
-
The following data for 2016 relate to Hay Industries, a worldwide conglomerate: REQUIRED: Answer the following questions related to Hay's required segment disclosures and show computations: 1. Which...
-
From this abbreviated description, what resources and capabilities do you think The Coca-Cola Company has? Does the fact that an organization is so heavily into global markets make it more difficult...
-
Consider the data in the Excel file Consumer Price Index. a. Use simple linear regression to forecast the data. What would be the forecasts for the next 2 years? b. Use the double exponential...
-
Suppose two firms want to borrow money from a bank for a period of one year. Firm A has excellent credit, whereas Firm Bs credit standing is such that it would pay prime + 2 percent. The current...
-
The Data file Median Household contains the median household income for a family with two earners for each of the fifty states (American Community Survey, 2013). a. Construct a frequency and a...
-
Di-peptides result from the reaction of two amino acids to form an amide. Explain which nitrogen of the following di-peptide is the stronger base: CH,O LI H_NCH, C-NH-CH-C-0
-
The pKa of the carboxylic acid group of acetic acid is 4.7. The pKa of the carboxylic acid group of the conjugate acid of the amino acid alanine is 2.3. Explain the difference in these pKa values....
-
What is discovery, and how does electronic discovery differ from traditional discovery?
-
In the circuit shown below, IE = 120 mA, a = 0.99, a2 = 0.98. Assuming that both transistors are in the active state, answer the following questions: 1. Find IC, IB, IE, IC, and Ic. 2. Find the...
-
Find the open intervals where the function is concave upward or concave downward. Find any inflection points. f(x)=-(x+1)6 Find any critical numbers for f and then use the second derivative test to...
-
Worked Example A two-dimensional incompressible flow is expressed by the velocity functions: u = -2xy y = y = x 1 w=0 Find the pressure field p(x, y) when the pressure at the origin (x=o, y=o) is p.....
-
The following information is available for Blue Spruce Corp. for 2022. Cash used to purchase treasury stock Cash dividends paid $111,592 50,576 Cash paid for interest Net income 51,968 1,077,176...
-
3 4pts Foam k MA C L H W F A box is transported by a truck. Suddenly, the driver uses the brakes and applies 100 N deceleration force to the box. The weight of the box is 20 Kg and its dimensions are...
-
Explain the usefulness of benchmarking energy consumption.
-
What is a manufacturing system?
-
Sketch the graph of the equation. x = 3
-
How might you prepare 2-phenylethanol from benzyl bromide? More than one steps inneeded. CH2Br CH2CH2OH
-
How might you carry out the following transformation? More than one step is needed. -CH2 CH2CH2OH
-
How would you prepare the following carbonyl compounds from anitrile? ( (a) CH3CH2CCH2CH3 CH O2N
-
Need help filling out these tax forms. Not sure how to do 1040 page 2 or schedule 3. I think I have schedule 1 right but need help with the itemized deductions for 1040 page 1 Required information...
-
Question:What should Airbus and Boing have learned from IBERIA case? What changed in the industry when Boing decided to develop Dreamliner in 2003?( Read the following case and ppt) Airline Route...
-
Which of the following needs to be always assessed when you are evaluating the literature you have obtained for your research? O All of the above O Sufficiency Value O Relevance Several approaches...

Study smarter with the SolutionInn App


