Question: Aqueous solutions of the amino acid L-isoleucine (Ile) are prepared by putting 100.0 grams of pure water into each of six flasks and adding different
Aqueous solutions of the amino acid L-isoleucine (Ile) are prepared by putting 100.0 grams of pure water into each of six flasks and adding different precisely weighed quantities of Ile to each flask. The densities of the solutions at 50.0 + 0.05oC are then measured with a precision densitometer, with the following results:
(a) Plot a calibration curve showing the mass ratio, r, as a function of solution density, p, and fir a straight line to the data to obtain an equation of the form r = ap + b.
(b) The volumetric flow rate of an aqueous Ile solution at a temperature of 50oC is 150L/h. The density of sample of the stream is measured at 50oC and found to be 0.9940g/cm3. Use the calibration equation to estimate the mass flow rate of Ile in the stream (kg Ile/h).
(c) It has just been discovered that the thermocouple used to measure the stream temperature was poorly calibrated and the temperature was actually 47oC. Would the Ile mass flow rate calculated in part (b) be too high or too low? State any assumption you make and briefly explain youreasoning.
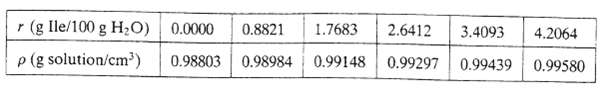
r (g Ile/100 g HO) 0.0000 0.8821 1.7683 p (g solution/cm) 0.98803 0.98984 2.6412 3.4093 4.2064 0.99580 0.99148 0.99297 0.99439
Step by Step Solution
3.44 Rating (170 Votes )
There are 3 Steps involved in it
a Conc g lle100 g H2O 45 ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
13-E-C-E-C-P (57).pdf
180 KBs PDF File
13-E-C-E-C-P (57).docx
120 KBs Word File


