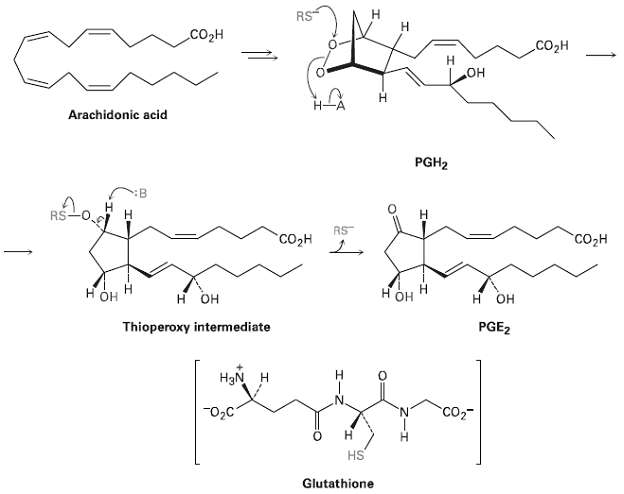
Assign R or S configuration to each chirality center in prostaglandin E2 (Figure) the most abundant and
Question:
Assign R or S configuration to each chirality center in prostaglandin E2 (Figure) the most abundant and biologically potent of mammalianprostaglandin
Transcribed Image Text:
н Н RS Соон .CO2H он Н Arachidonic acid PGH2 H. RS RS "СОдн "Соон нон он H OH OH PGE2 Thioperoxy intermediate HgN H "02C CO2 н HS Glutathione
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (15 reviews)
R H I R ...View the full answer

Answered By

Michael Owens
I am a competent Software Engineer with sufficient experience in web applications development using the following programming languages:-
HTML5, CSS3, PHP, JAVASCRIPT, TYPESCRIPT AND SQL.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Assign R or S configuration to each chirality center in the followingmolecules: (b) NH2 (a) . "C r "O
-
Assign R or S configuration to each chirality center in the following monosaccharide?s and tell whether each is a D sugar or an L sugar: (b) (a) - (c) - - - - - - - - - - CH- CH2 CH2
-
Assign R or S configuration to each chirality center in the followingmolecules: (a) (b) ,CH (c) , . CH CHCH
-
The management accountant is preparing the master budget for her retail firm. The following information has been supplied Sales $300,000 Opening inventory $40,000 Closing inventory $60,000 Required...
-
How does an agenda help make a meeting more successful?
-
the given matrix is of the form In each case, A can be factored as the product of a scaling matrix and a rotation matrix. Find the scaling factor r and the angle of rotation. Sketch the first four...
-
4. Dees consultant was given permission to install software on a server inside Emersons network. Suppose he had been a computer criminal. a. Using Figure 12-1 as a guide, what might he have done? b....
-
What other strategies might help organizations better utilize and manage selection activities?
-
Data about the marital status, withholding allowances, and weekly salaries of the four office workers at Peter Office Supply Company follow. (Use the tables shown in Figure 10.2a & Figure 10.2b.)...
-
Use Solver to determine the weights for a four-period weighted moving average on the data set that minimizes the MSE. a. What are the optimal values for the weights? b. Prepare a line graph comparing...
-
Write the saponification reaction of glyceryl di-oleate monopalmitate with aqueous NaOH.
-
Studies of the conversion of mevalonate 5-phosphate to isopentenyl diphosphate have shown the following result. Which hydrogen, pro-R or pro-S, ends up cis to the methyl group, and which ends...
-
Draw the hierarchy chart and design the logic for a program that calculates the projected cost of a remodeling project. Assume that the labor cost is $30 per hour. Design a program that prompts the...
-
Perhaps we need a way to differentiate ourselves from the competition? Is it possible that we are dividing the customer's time too much? Does this mean that we should instead look to attract more...
-
Complete these answers with full paragraph sentences. 1)What are the Mission, Vision, & Values of the Palo Alto Network? 2) What are the Four Functions of Management Planning, Organizing, Leading, &...
-
One highly visible trait of a successful leader is that of role model: behavior exhibited by a leader is carefully observed and often sets the tone for the entire center. As a role model, it is...
-
Design a flowchart that illustrates the key processes and decision points within the custom leadership system, along with the various inputs and outputs. At the center of the flowchart is the leader,...
-
Prepare a sample memo to those that have been selected to serve on the "Bulletin 1" committee. Remind them of their charge and outline a calendar of meetings. Lastly, include a list of resources. ...
-
What is a career path? LO.1
-
Find the center of mass of a thin triangular plate bounded by the y-axis and the lines y = x and y = 2 - x if (x, y) = 6x + 3y + 3.
-
A 0.148 M solution of a monoprotic acid has a percent ionization of 1.55%. Determine the acid ionization constant (K a ) for the acid.
-
Describe the 1H NMR spectrum of a. BrCH2CH2Cl b. ClCH2CH2Cl
-
Write an equation for the reaction of pyridine with a. cold sulfuric acid (H2SO4) b. cold nitric acid (HNO3)
-
Although nitration of pyridine requires a temperature of 300°C (eq. 13.2), 2,6 dimethylpyridine is readily nitrated at 100°C. Write an equation for the reaction, and explain why milder...
-
Current Attempt in Progress On July 3 1 , 2 0 2 2 , Crane Compary had a cash balance per books of $ 6 , 2 4 5 . 0 0 . The statement from Dakata State Bark on that date showed a balance of $ 7 , 7 9 5...
-
Cede & Co. expects its EBIT to be $89,000 every year forever. The firm can borrow at 5 percent. Cede currently has no debt, and its cost of equity is 10 percent. If the tax rate is 35 percent, what...
-
In the Marriott example, one discussion point considered when a firm might use a single hurtle rather than different divisional or business unit rates. When a single rate is used and the divisions...

Study smarter with the SolutionInn App


