Assign stereochemistry (E or Z) to the double bonds in each of the following compounds, and convert
Question:
Assign stereochemistry (E or Z) to the double bonds in each of the following compounds, and convert each drawing into a skeletal structure (red ? 0, yellow-green = C1):
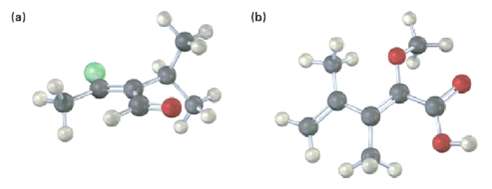
Transcribed Image Text:
(b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 63% (11 reviews)
3 High Cl Lo...View the full answer

Answered By

Charles mwangi
I am a postgraduate in chemistry (Industrial chemistry with management),with writing experience for more than 3 years.I have specialized in content development,questions,term papers and assignments.Majoring in chemistry,information science,management,human resource management,accounting,business law,marketing,psychology,excl expert ,education and engineering.I have tutored in other different platforms where my DNA includes three key aspects i.e,quality papers,timely and free from any academic malpractices.I frequently engage clients in each and every step to ensure quality service delivery.This is to ensure sustainability of the tutoring aspects as well as the credibility of the platform.
4.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Each of the following compounds has been prepared from p-nitroaniline. Outline a reasonable series of steps leading to each one. (a) p-Nitrobenzonitrile (d) 3, 5-Dibromoaniline (b) 3, 4,...
-
Each of the following compounds has been prepared from o-anisidine (o-methoxyaniline). Outline a series of steps leading to each one. (a) o-Bromoanisole (d) 3-Fluoro-4-methoxybenzonitrile (b)...
-
Each of the following compounds is incorrectly named. What is wrong with each name, and what is the correct name for each compound? a. FeCl3, iron chloride b. NO2, nitrogen(IV) oxide c. CaO,...
-
In 2017, Barlow moved from Chicago to Miami to start a new job, incurring costs of $1,200 to move household goods and $2,500 in temporary living expenses. Barlow was not reimbursed for any of these...
-
Why would being a highly narcissistic, charismatic person interfere with being an effective servant leader?
-
Discuss macroeconomic factors that would influence the yield curve.
-
How does the U.S. tax structure influence a firms willingness to finance with debt? AppendixLO1
-
1. What factors should be considered when developing inventory systems for the 10,000 items carried by Dano's? What are the key differences between items that would affect how their inventory is...
-
Ginger Baker, Inc. just paid an annual dividend of $3 a share and this dividend is expected to grow at a rate of 4% per year for the foreseeable future. If the discount rate for Ginger Baker is 9%,...
-
Refer to the facts in problem 6. Now assume that Firm A borrowed $50,000 to purchase the asset. In each year, it paid $3,800 annual interest on the debt. The interest payments were deductible. a. How...
-
Name the following alkenes, and convert each drawing into a skeletal structure: (b) (a)
-
The following carbocation is an intermediate in the electrophilic addition reaction of 1-ICI with two different alkenes. Identify both, and tell which C?IT bonds in the carbocation are aligned for...
-
Go to www.numa.com and look under the section titled Options and follow the calculator link. You purchased a call option for $10.50 that matures in 51 days. The strike price is $100, and the...
-
Use the Comprehensive Annual Financial Report for the Village of Arlington Heights (please look up this content) for the year ended December 31, 2018, to answer questions 8-20. All questions are on...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
Two wires lie perpendicular to the plane of the screen and carry equal magnitudes of electric current in the directions shown. Point P is equidistant from the two wires. The distance between each of...
-
Your firm has recently been appointed as auditors of Kentronics Ltd , a large company which markets sophisticated electronic equipment for heavy industry as well as the mining equipment industry. The...
-
The pulse rates of 152 randomly selected adult males vary from a low of 37 bpm to a high of 117 bpm. Find the minimum sample size required to estimate the mean pulse rate of adult males. Assume that...
-
Define the following concepts as they relate to sexual harassment. Zero tolerance Prevention Investigation Resolution
-
Refer to the Conservation Ecology (Dec. 2003) study of the causes of forest fragmentation, presented in Exercise 2.166 (p. 97). Recall that the researchers used advanced high-resolution satellite...
-
Suppose that 0.95 g of water condenses on a 75.0-g block of iron that is initially at 22 C. If the heat released during condensation goes only to warming the iron block, what is the final temperature...
-
Which compound in each set should have the larger dipole moment? Explain. Propene or 2-methylpropene
-
(a) If the standard enthalpy change for the reaction 2-ethyl- I -butene I -hexene is + I 5.3 kJ mol-r (+3.66 kcal mol-t). and if Afli for l-hexere is -40.5 kJ mol-r (-9.68 kcal mol-1 2-methy...
-
Within each series arrange the compounds in order of increasing stability: C(CH3)3 HO CH,CH(CH)
-
Comparative financial statements for Weller Corporation, a merchandising company, for the year ending December 31 appear below. The company did not issue any new common stock during the year. A total...
-
Mrquered Mrquered
-
You plan to invest $10,00 today in an investment account earning 5% interest. You then plan to invest an additional $1,000 into this account each year for the next twenty years. How much money will...

Study smarter with the SolutionInn App


