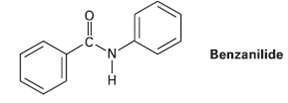
At what position, and on what ring, would you expect bromination of benzanilide to occur? Explain by
Question:
At what position, and on what ring, would you expect bromination of benzanilide to occur? Explain by drawing resonance structures of theintermediates.
Transcribed Image Text:
Benzanilide 'N' H.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
00 1z Deactivated Activated by C0 byN Br2 FeBr3 H 00 Br Br ...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
By drawing resonance structures for the carbocation intermediates, show why aromatic substitution in pyridine - N - oxide occurs at the 4 - position rather than at the 3 - position.
-
At what position and on what ring do you expect nitration of 4-bromo- biphenyl to occur? Explain, using resonance structures of the potentialintermediates. 4-Bromobiphenyl Br
-
Would you expect the reactivity of a five-membered ring ether such as tetrahydrofuran (Table 12.2) to be more similar to an epoxide or to a noncyclic ether?
-
What is your perspective on changes in cultural ideals for the female body from that of Marilyn Monroe in the 1950s to top models today?
-
Rex and Agnes Harrell purchased a beach house at Duck, North Carolina, in early 2015. Although they intended to use the beach house occasionally for recreational purposes, to help pay the mortgage...
-
Discuss requirements of the Truth-in-Lending Act as it relates to finance charges for mortgage loans?
-
A company has net income of $300,000, net sales of $2,500,000, and total assets of $2,000,000. Its return on total assets equals: a. 6.7% b. 12.0% c. 8.3% d. 80.0% e. 15.0% AppendixLO1
-
Little Oil has outstanding 1 million shares with a total market value of $20 million. The firm is expected to pay $1 million of dividends next year, and thereafter the amount paid out is expected to...
-
First of all, we need to choose one of these options to answer the question 1 Barbour Corporation, located in Buffalo, New York, is a retailer of high-tech products and is known for its excellent...
-
1. using the Whirlpool Corporation Web Site (www.whirlpoolcorp.com), identify the employee network groups at Whirlpool and the mission of each. 2. Do you think Whirlpools encouragement of employee...
-
At what position, and on what ring, would you expect the following sub stances to undergo electrophilicsubstitution? (a) (b) CH3 Br (c) (d) .CI CH3 Z O=U
-
Would you expect the FriedelCrafts reaction of benzene with (R)-2-chloro- butane to yield optically active or racemic product explain.
-
Which one of the following graphs correctly represents Ohm's law, where V is the voltage and I is the current? (a) A (b) B (c) C (d) D A.
-
As of June 30, 2012, the bank statement showed an ending balance of \(\$ 13,879.85\). The unadjusted Cash account balance was \(\$ 13,483.75\). The following information is available: 1. Deposit in...
-
An engineering study has developed the following cost data for the production of product A: (1) If the current production level is 1,500 units, what is the incremental cost of producing an additional...
-
On December 1, a group of individuals formed a corporation to establish the Local, a neighborhood weekly newspaper featuring want ads of individuals and advertising of local firms. The free paper...
-
Design an arithmetic circuit with one selection variable S and two n-bit data inputs A and B. The circuit generates the following four arithmetic operations in conjunction with the input carry C in ....
-
For the system you chose for Problems and Exercises 3, complete section 4.0, A-C, Management Issues, of the BPP Report. Why might people sometimes feel that these additional steps in the project plan...
-
What would it be like to work at Patagonia? (Hint: Go to Patagonias Web site and find the section on jobs.) Whats your assessment of the companys work environment?
-
The manager for retail customers, Katie White, wants to hear your opinion regarding one business offer she has received from an entrepreneur who is starting a mobile phone app called Easy Money. The...
-
Redo Problem 5.22 using Aspen Plus. Problem 5.22 Isobutane is to be liquefied to make liquid petroleum gas (LPG). The butane is available at 25 C and 1 bar, it will be compressed to 15 bar, cooled...
-
Show syntheses of these compounds from1-bromobutane: b) TH. c)
-
Show the products of thesereactions: CH CH;CH C3D CH CH3 .. -- NaOH CH3
-
Explain how a similar hydroboration reaction could be used to prepare (R)-2-butanol in good enantiomeric excess.
-
FINANCIAL STATEMENT ANALYSIS INSTRUCTIONS 1. PREPARE RATIO ANALYSIS REPORT ( word file) Format 1. Introduction 2. Importance of Financial Statements 3. Importance of Financial statement analysis and...
-
Let us assume that Europe is in recession, China's economy is slowing down, and the US economy is growing at 1-2%. Use these assumptions to invest in 4 ETFs (electronically traded funds). The 4 ETFs...
-
A section 83(b) election creates ordinary income at the time of the grant. Ture or False

Study smarter with the SolutionInn App


