At what position, and on what ring, would you expect the following sub stances to undergo electrophilicsubstitution?
Question:
At what position, and on what ring, would you expect the following sub stances to undergo electrophilicsubstitution?
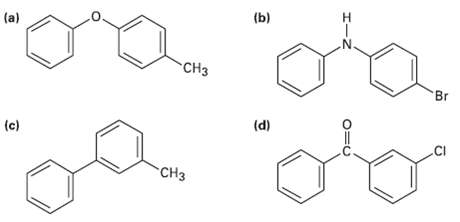
Transcribed Image Text:
(a) (b) CH3 Br (c) (d) .CI CH3 エーZ O=U
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (15 reviews)
ogad CH3 Activated by0 Activated byO and CH3 Activated byN Substitutio...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
At what position and on what ring do you expect nitration of 4-bromo- biphenyl to occur? Explain, using resonance structures of the potentialintermediates. 4-Bromobiphenyl Br
-
Would you expect the following compound to be aromatic? Justify your answer. OR N-
-
What effect would you expect the use of MACRS depreciation rules to have on the acceptability of a project having a 10-year economic life but a 7-year MACRS classification?
-
How would this photo be different if the two people were both Americans?
-
Mona viewed herself as a creative individual who had chosen to go to law school for economic reasons. Mona's undergraduate majors were creative writing and American Indian studies. Mona was very...
-
Refer to Exercise 1. Develop an opportunity loss table. Determine the opportunity loss for each decision. In exercise 1 The following payoff table was developed. Let P(S 1 ) = .30, P(S 2 ) = .50, and...
-
Mozart Co. owns 35% of Melody Inc. Melody pays $50,000 in cash dividends to its shareholders for the period. Mozarts entry to record the Melody dividend includes a: a. Credit to Investment Revenue...
-
Is there a difference in the variation of the yields of different types of investments? The file CD Rate contains the yields for one- year certificates of deposit (CDs) and five- year CDs for 22...
-
Jacob inherited a farm his grandfather had purchased 50 years ago for $40,000. It was valued at $1,000,000 when his grandfather died but has a current value of $1,100,000. Jacob did not like farming...
-
Jimmy owns a garden in which he has planted N trees in a row. After a few years, the trees have grown up and now they have different heights. Jimmy pays much attention to the aesthetics of his...
-
Triphenylmethane can be prepared by reaction of benzene and chloroform in the presence of A1Cl3. Propose a mechanism for thereaction. H. + CHCI3 AICI3
-
At what position, and on what ring, would you expect bromination of benzanilide to occur? Explain by drawing resonance structures of theintermediates. Benzanilide 'N' H.
-
Calculate [H 3 O + ], [HSO 4 - ], and [SO 4 2- ] in (a) 0.75 M H 2 SO 4 ; (b) 0.075 M H 2 SO 4 ; (c) 7.5 x 10 -4 M H 2 SO 4 .
-
Prove that Eq. (19.34) gives the simplest multi-gluon and gluon-quark states that contain an \(\mathrm{SU}(3)\) color singlet in the decomposition. Data from Eq. 19.34 (GG)1: (88)1 (Gqq) : [8 (383)8]...
-
In question 70, what is the probability that of the 100 cars test-driven, more than 35 cars get more than 45 miles per gallon? How many of the 100 cars tested would you expect to get more than 45...
-
Construct the braid group products (a) (b) using the algorithm of Fig. 29.16 . Data from Fig. 29.16
-
Worksheet The adjusted trial balance columns of a worksheet for Bond Corporation are shown below. The worksheet is prepared for the year ended December 31. Complete the worksheet by (a) entering the...
-
The Healthy Catering Service had the following transactions in July, its first month of operations: 1 Kelly Foster contributed \(\$ 18,000\) of personal funds to the business in exchange for common...
-
Do you think pay-for-performance programs really work?
-
APC16550D UART has a clock running at18.432 MHz and its baud rate is set to 2000.Determine the HEX contents of its DLM and DLL registers. Please can you explain step by step and in detail how you get...
-
Redo Problem 5.23 using Aspen Plus. Problem 5.23 Repeat the calculation of problem 5.22 with the vapor being recycled to the compressor Problem 5.22 Isobutane is to be liquefied to make liquid...
-
This hydroboration reaction forms two products. Show these products and explain which one you expect to be amajor. 1) BH3, THF 2) H,O2. NAOH
-
Show preparation of these alcohols fromalkenes. b) c)
-
Show the products of thesereactions: 1) BH3, THF 2) H2O2, NaOH a) Ph- b) 1) disiamylborane 2) HO2, NaOH
-
During the month of September,the Cider Pressing Company is trying to determine how much cider they are going to sell in October and November. One gallon of cider typically sells for $7 per gallon....
-
This is very confusing please help with descriptions if possible. Complete this question by entering your answers in the tabs below. Prepare a master budget for the three-month period ending June 30...
-
Doug recibe un dplex como regalo de su to. La base del to para el dplex y el terreno es de $90,000. En el momento de la donacin, el terreno y el edificio tienen un FMV de $40 000 y $80 000,...

Study smarter with the SolutionInn App


