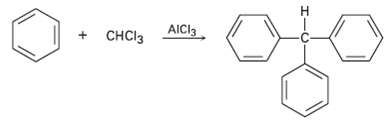
Triphenylmethane can be prepared by reaction of benzene and chloroform in the presence of A1Cl3. Propose a
Question:
Triphenylmethane can be prepared by reaction of benzene and chloroform in the presence of A1Cl3. Propose a mechanism for thereaction.
Transcribed Image Text:
H. + CHCI3 AICI3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
CHCl3 AICI3 CHC CHCI2 CHCI AICI4 CHC1 CIAI Cl3 D...View the full answer

Answered By

Sumit kumar
Education details:
QUATERNARY Pursuing M.Tech.(2017-2019) in Electronics and Communication Engg. (VLSI DESIGN) from
GNIOT Greater Noida
TERTIARY B.Tech. (2012-2016) in Electronics and Communication Engg. from GLBITM Greater Noida
SECONDARY Senior Secondary School Examination (Class XII) in 2012 from R.S.S.Inter College, Noida
ELEMENTARY Secondary School Examination (Class X) in 2010 from New R.J.C. Public School ,Noida
CERTIFICATION
Summer Training in ‘WIRELESS EMBEDDED SYSTEM’ from ‘XIONEE’ for the six weeks.
EMBEDDED SYSTEM Certificate issued by CETPA INFOTECH for one day workshop.
Certificate of Faculty development program on OPTICAL COMMUNICATION and NETWORKS for one week.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The following alkyl bromide can be prepared by reaction of the alcohol (S)-2-pentanol with PBr3. Name the compound, assign (R) or (S) stereochemistry, and tell whether the reaction of the alcohol...
-
Ethers can be prepared by reaction of an alkoxide or phenoxide ion with a primary alkyl halide. Anisole, for instance, results from reaction of sodium phenoxide with iodomethane. What kind of...
-
Norbornadiene (Problem 14.41) can be prepared by reaction of chloroethylene with 1, 3-cyclopentadiene, followed by treatment of the product with sodium ethoxide. Write the overall scheme, and...
-
How would you compare the value of direct definition from friends in face-to-face conversation and strangers comments on a YouTube posting?
-
The $1 million maximum compensation deduction does not seem to have deterred large corporations from remunerating their executives at very high levels. What techniques are being used to work around...
-
The U.S. Department of Transportation estimates that 10% of Americans carpool. Does that imply that 10% of cars will have two or more occupants? A sample of 300 cars traveling southbound on the New...
-
Earlier this period, Amadeus Co. purchased its only availablefor- sale investment in the stock of Bach Co. for $83,000. The period-end market value of this stock is $84,500. Amadeus records a: a....
-
Rishi Singh has $1,500 to invest. His investment counselor suggests an investment that pays no stated interest but will return $2,000 at the end of 3 years. a. What annual rate of return will Rishi...
-
Accounting Information System Identify spesific name of the edit check omitted or incorporated in the following facts: a. A G/L clerk omitted credit amount from a journal entry but the transaction...
-
1. Buckley, a worker in a restaurant, stole a credit card from the coat of its owner with the intention of using it to charge goods. He purchased some merchandise at a retail store and paid for these...
-
Addition of HBr to 1-phenyipropene yields only (1-bromopropyl) benzene. Propose a mechanism for the reaction, and explain why none of the other regioisomer isproduced. Br + HBr
-
At what position, and on what ring, would you expect the following sub stances to undergo electrophilicsubstitution? (a) (b) CH3 Br (c) (d) .CI CH3 Z O=U
-
The information below and on page 1194 was disclosed during the audit of Elbert Inc. 1. 2. On January 1, 2012, equipment costing $600,000 is purchased. For financial reporting purposes, the company...
-
(a) Draw a simplified ray diagram showing the three principal rays for an object located inside the focal length of a converging lens, closer to the lens than to the focal point. (b) Is the image...
-
Power efficiency has become very important for modern processors, particularly for embedded systems. Create a version of gcc for two architectures that you have access to, such as x86, RISC-V,...
-
There is a movement toward wireless mobile computing using thin-client technology. Go to the Web and visit some of the ma jor computer vendors that are producing thin-client products such as handheld...
-
Draw a B-tree of order 4 and height 3 containing the fewest elements. Show an example of a split that would be applied by inserting the fewest number of elements.
-
Repeat Example 10-4, except calculate the diameter at the bottom of the column. Example 10-4 A distillation column is separating n-hexane from n-heptane using 1-in. ceramic Intalox saddles. The...
-
Look back at the statement made by the SAS employee on the Best Companies survey. What does that tell you about the importance of understanding individual behavior?
-
In a nonmagnetic medium, E = 50 cos (10 9 t 8x) a y + 40 sin (10 9 t 8x) a z V/m find the dielectric constant r and the corresponding H.
-
Redo Problem 5.27 using Aspen Plus. Problem 5.27 Methane at 260 K is to be isothermally compressed from 0.1 MPa to 1.0 MPa. a. What is the minimum work required, and how much heat must be removed to...
-
Show the products of thesereactions: 1) Hg(O,CCH,) . 2) NaBH3. NAOH 1) Hg(O,CCH,)2, H,0 2) NaBH4, NaOH b) a) . H SO4 . H2SO4 d) HgSO, H9SO,
-
Explain which of the reaction would provide a better synthesis of3-hexanone. . . CH,CH,CH,CH,CH, 3-Hexanone CH,CH,C=CCH CH3 CH,C=CCH,CH,CH3 H,SO4 HgSO, H&SO,
-
Show the products of thesereactions: 1) BH3, THF 1) BH3. THF 2) .. NaOH b) 2) ,, NaOH 1) BH3, THF 2) H,O2. NaOH CH-CH3
-
A government bond matures in 30 years, makes semi-annual coupon payments of 6.0% ($120 per year) and offers a yield of 3.7% annually compounded. Assume face value is $1,000. Three years later the...
-
Your objective is: 1. Carry out a life insurance needs analysis, for each one of them (show your calculations) [30 Marks] 2. Refer to the case and the insurance plan quotes. Would you recommend...
-
TufStuff, Incorporated, sells a wide range of drums, bins, boxes, and other containers that are used in the chemical industry. One of the company s products is a heavy - duty corrosion - resistant...

Study smarter with the SolutionInn App


