Show the products of thesereactions: 1) Hg(O,CCH,) . 2) NaBH3. NAOH 1) Hg(O,CCH,)2, H,0 2) NaBH4, NaOH
Question:
Show the products of thesereactions:
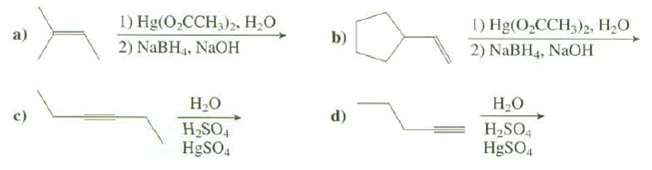
Transcribed Image Text:
1) Hg(O,CCH,)» Н.О 2) NaBH3. NAOH 1) Hg(O,CCH,)2, H,0 2) NaBH4, NaOH b) a) Н.о H SO4 Н.о H2SO4 d) HgSO, H9SO,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (12 reviews)
a...View the full answer

Answered By

Susan Juma
I'm available and reachable 24/7. I have high experience in helping students with their assignments, proposals, and dissertations. Most importantly, I'm a professional accountant and I can handle all kinds of accounting and finance problems.
4.40+
15+ Reviews
45+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Show the products of these reactions. (Remember that acid-base reactions are usually much faster than nucleophilic substitution reactions.) a) C1-CHCHCHCOH b) Br OH NH3 + OH
-
Show the products of these reactions and explain whether each would follow an SN1 or an SN2 mechanism: a) C) B CI + OH Br + SH DMF CHOH HO Br + HO CHOH HO b) d) f) CI + HO CH,OH OTS + CH0 Br + CHCO...
-
Show the products of these eliminationreactions: CH3 ELOH + NaOCH,CH3 a) "CI Br ELOH + NaOCH,CH; b) "CH,CH
-
Calculate the density of N2 at STP, (a) using the ideal-gas law and (b) using the molar volume and molar mass of N2. How do the densities compare?
-
Wilson revises her estimates of the benefits from the new system's lower labor costs as calculated in P1-28A. She now thinks the savings will be only $935,000. Requirements 1. Compute the expected...
-
A Remington School District employee has been charged with theft and forgery for allegedly stealing approximately $72,000 in district funds. Mary Blaner, 51, was charged in District Court with one...
-
Refer to Exercise 9.10. After the least squares line has been obtained, the table below (which is similar to Table 9.4) can be used for (1) comparing the observed and the predicted values of y, and...
-
An economist reports that 560 out of a sample of 1,200 middle-income American households actively participate in the stock market. a. Construct the 90% confidence interval for the proportion of...
-
An unexpected decrease in market interest rates will cause a Question 20 options: fixed-rate bond's coupon rate to decrease zero coupon bond's current yield to decrease coupon bond's current yield to...
-
Please solve this problem using C language Hacker Industries has a number of employees. The company assigns each employee a numeric evaluation score and stores these scores in a list. A manager is...
-
Show all the steps in the mechanism for the formation of MTBE from methanol and isobutylene.
-
Explain which of the reaction would provide a better synthesis of3-hexanone. . . CH,CH,CH,CH,CH, 3-Hexanone CH,CH,C=CCH CH3 CH,C=CCH,CH,CH3 H,SO4 HgSO, H&SO,
-
It is claimed that the following inequality is true for all negative numbers x and y: The following proof is offered by a student: a. Explain the error made by the student. b. By use of a...
-
2.11.2Project:Performance Task: The Parallax Problem Project Geometry Sem 1 (S3537251) Julio Duenas Points possible:120 Date: ____________ The Scenario:You're looking for a sponsor to pay for you to...
-
If the most common treatment of assigning overapplied overhead was used, the final balance in Cost of Goods Sold would have been * (1 Point) At the end of the last fiscal year, BREAD Company had the...
-
Angelina received new word processing software for her birthday. She also received a cheque with which she intends to purchase a new computer. Angelina's UNILUS Professor assigned a paper due in two...
-
At date t, the portfolio P to be hedged is a portfolio of Treasury bonds with various possible maturities. Its characteristics are as follows: Value YTM MD Convexity $1,450 6% 4.25 55 We consider...
-
A playground merry-go-round with an axis at the center (radius R = 1.3 m and rotational inertia | = 1.2 x 103 kgm2) is initially rotating at angular velocity w = 0.21 rad/s clockwise). A girl of mass...
-
What percentage of the employees in a company must sign authorization cards before a union certification election can be called?
-
As indicated by mutual fund flows, investors tend to beat the market seek safety invest in last year's winner invest in last years loser
-
Calculate the value of the equilibrium constant for each of the following reactions in aqueous solution. a. HCHO + OHCHO + HO b. C,H,O, +H*
-
Predict the product(s) of the following reactions. If more than one product is formed, tell which ismajor. (a) CH3I (excess) Ag20, H20 eat C? A? B? .cocI (b) H20 NaN3 eat C? B? A? (c) CgHsCH2Br C7...
-
Fill in the missing reagents a?c in the following scheme: NH2 CHCH3 CH=CH2 CCH3 b, c CHCH2NCH3 CH CH-CH2
-
Although pyrrole is a much weaker base than most other amines, it is a much stronger acid (pK a 15 for the pyrrole versus 35 for diethyl amine). The NH proton is readily abstracted by base to yield...
-
Suppose you took a long position on a put option with an exercise price of $2.15 per pound and paid a premium of $0.24 per pound. Required: If the spot exchange rate turns out to be $2.30 per pound...
-
Youve observed the following returns on Crash-n-Burn Computers stock over the past five years: 15 percent, 6 percent, 18 percent, 14 percent, and 10 percent. Suppose the average inflation rate over...
-
Required : a- outline the statement of comperhensive income for the year ended 30 november 2021 b- outline the statment of financial position as at 30 November The Trial Balance of Alim Enterprise at...

Study smarter with the SolutionInn App


