Question: Explain which of the reaction would provide a better synthesis of3-hexanone. . . CH,CH,CH,CH,CH, 3-Hexanone CH,CH,C=CCH CH3 CH,C=CCH,CH,CH3 H,SO4 HgSO, H&SO,
Explain which of the reaction would provide a better synthesis of3-hexanone.
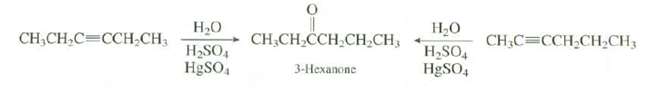
. . CH,CH,CH,CH,CH, 3-Hexanone CH,CH,C=CCH CH3 CH,C=CCH,CH,CH3 H,SO4 HgSO, H&SO,
Step by Step Solution
3.44 Rating (179 Votes )
There are 3 Steps involved in it
The left synthesis starting with 3hex... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
15-C-O-C (151).docx
120 KBs Word File


