Oxazole is a live-membered aromatic heterocycle. Would you expect oxazole to be more basic or less basic
Question:
Oxazole is a live-membered aromatic heterocycle. Would you expect oxazole to be more basic or less basic than pyrrole?Explain.
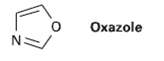
Transcribed Image Text:
Oxazole N:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (14 reviews)
B sze H Oxazole Oxazole is an aromatic 6a electron heterocycle Two oxygen electrons and one nitrogen electron ...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Would you expect heteroscedasticity to be present in the following regressions? Sample Net worth Log of net worth (a) Corporate profits Fortune 500 (b) Log of corporate (c) Dow Jones industria (o)...
-
Would you expect lysozyme to hydrolyze cellulose? Why or why not?
-
Would you expect insulin to increase or decrease the activity of the enzyme ATP-citrate lyase?
-
Kellogg Company is expected to pay $2.00 in annual dividends to its common shareholders in the future. Our best estimate of the expected cost of equity capital is 5.0% and the expected growth rate in...
-
How do talent management evidence-based HR support Mundy's efforts to offer solutions?
-
A helium-neon laser ( = 633 nm) illuminates a single slit and is observed on a screen 1.5 m behind the slit. The distance between the first and second minima in the diffraction pattern is 4.75 mm....
-
What, if any, general assistance can his organization headquartered in the United States give him as he addresses these concerns?
-
Assume that Santana Rey expands Business Solutions accounting system to include special journals. Required 1. Locate the transactions related to January through March 2012 for Business Solutions in...
-
Question 19 (1 point) Pearson Company bought a machine on January 1, 2022. The machine cost $180,000 and had an expected salvage value of $30,000. The life of the machine was estimated to be 5 years....
-
Histamine, whose release in the body triggers nasal secretions and constricted airways, has three nitrogen atoms. List them in order of increasing basicity, and explain yourordering. NH2 Histamine H.
-
Protonation of an amide using strong acid occurs on oxygen rather than on nitrogen. Suggest a reason for this behavior, taking resonance intoaccount. :0: H2SO4 NH2 NH2 R.
-
In Problem find each derivative and simplify. d -log2(3x2- dx 1)
-
Indicate whether each of the following types of transactions will either (a) increase stockholders' equity or (b) decrease stockholders' equity: 1. expenses 2. revenues 3. stockholders' investments...
-
The following selected transactions were completed by Lindbergh Delivery Service during October: 1. Received cash from issuing capital stock, \($75,000\). 2. Paid rent for October, \($4,200\). 3....
-
Murray Kiser operates his own catering service. Summary financial data for February are presented in equation form as follows. Each line designated by a number indicates the effect of a transaction...
-
A. Given that y = e 2x + 1 complete the table of values of y corresponding to x = 0.5, 1 and 1.5. B. Use the trapezium rule, with all the values of y in the completed table, to obtain an estimate for...
-
Draw a schematic using NFETs and PFETs for a restoring logic gate that implements the function = 0 if zero or two of inputs cba are true. Assume that all inputs and their complements are available.
-
Since it is unlikely that Erica Stovall will ask Mr. Jacobson whether or not Lindsey is a thief, should Jacobson volunteer this information? If so, how should he present this information? If not,...
-
The figure shows six containers, each of which is filled from the top. Assume that water is poured into the containers at a constant rate and each container is filled in 10 seconds. Assume also that...
-
Both H 2 O and H 2 PO 4 are amphoteric. Write an equation to show how each substance can act as an acid and another equation to show how each can act as a base.
-
From the dichlorination of propane, four isomeric products with the formula C3H6Cl2 were isolated and designated A, B, C, and D. Each was separated and further chlorinated to give one or more...
-
Write all of the steps in the free-radical chain mechanism for the monochlorination of ethane. What trace by-products would you expect to be formed as a consequence of the chain terminating steps?...
-
Using methanol (CH3OH) as a fuel source, write a balanced reaction for the formation of H2. Do the same for C10H22 (a component of diesel fuel). For an H2 fuel cell, why would it be better to use...
-
How do warehouses and distribution centers differ? What is cross-docking and why might a company choose to cross-dock a product? What kinds of products can be delivered electronically? What kinds...
-
Strawberry Inc. has historically been an all-equity firm. The analyst expects EBIT to be $1.5B in perpetuity starting one year from now. The cost of equity for the company is 11.5% and the tax rate...
-
Guzman company received a 60- day, 5 % note for 54,000 dated July 12 from a customer on account. Determine the due date on note. Determine the maturity value of the note and journalize the entry of...

Study smarter with the SolutionInn App


