Protonation of an amide using strong acid occurs on oxygen rather than on nitrogen. Suggest a reason
Question:
Protonation of an amide using strong acid occurs on oxygen rather than on nitrogen. Suggest a reason for this behavior, taking resonance intoaccount.
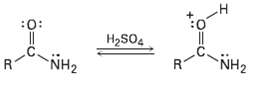
Transcribed Image Text:
:0: H2SO4 NH2 NH2 R.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (17 reviews)
0 NH3 NH NProtonation ...View the full answer

Answered By

Madhur Jain
I have 6 years of rich teaching experience in subjects like Mathematics, Accounting, and Entrance Exams preparation. With my experience, I am able to quickly adapt to the student's level of understanding and make the best use of his time.
I focus on teaching concepts along with the applications and what separates me is the connection I create with my students. I am well qualified for working on complex problems and reaching out to the solutions in minimal time. I was also awarded 'The Best Tutor Award' for 2 consecutive years in my previous job.
Hoping to get to work on some really interesting problems here.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
An equimolar mixture of oxygen and nitrogen enters a compressor operating at steady state at 10 bar, 220 K with a mass flow rate (m) of 1 kg/s. The mixture exits the compressor at 60 bar, 400 K with...
-
A friend of yours has performed three titrations: strong acid with a strong base, weak acid with a strong base, and weak base with a strong acid. He hands you the three titration curves, saying he...
-
Using the curved-arrow notation, derive a resonance structure for the allyl anion (shown here) which shows that the two carbon-carbon bonds an identical bond order of 1.5 and that the unshared...
-
You recently joined an accounting consulting firm. Your first client is a new manufacturing start-up that is trying to set up their costing system, and develop good processes for their upcoming...
-
What would you describe as Costco's basic strategy as a retailer? How do human resource practices support that strategy?
-
A Michelson interferometer is set up to display constructive interference (a bright central spot in the fringe pattern of Figure 33.25) using light of wavelength λ. If the wavelength is...
-
How can Charles deal with these issues in a way that is acceptable for both the organization and the employees?
-
The Index of Small Business Optimism declined 3 points in June 2012, falling to 91.4 and losing gains achieved earlier in the year, according to the NFIB Research Foundation report. Labor market...
-
Your company plans to issue bonds later in the upcoming year. But with the economic uncertainty and varied interest rates, it is not clear how much money the company will receive when the bonds are...
-
Read the Scenario Congratulations, you are now the Police Chief in Anytown, USA. A city with 30,000 residents and you are responsible to provide 24 hour a day police coverage. You have a total of 45...
-
Oxazole is a live-membered aromatic heterocycle. Would you expect oxazole to be more basic or less basic than pyrrole?Explain. Oxazole N:
-
Substituted pyrroles are often prepared by treatment of a 1, 4-diketone with ammonia. Propose amechanism. R + H20 RCCH2CH2R" R- NH3 R.
-
Selected condensed data taken from a recent balance sheet of Heidebrecht Inc. are as follows. HEIDEBRECHT INC. Balance Sheet (partial) Cash ............. $ 8,041,000 Short-term investments ......
-
Given forecast errors of 4, 8, and -3, what is the MAD? What is the MSE?
-
Padgett Rentals can purchase a van that costs \($48,000\) ; it has an expected useful life of three years and no salvage value. Padgett uses straight-line depreciation. Expected revenue is...
-
Rainwater Corp. expects to sell 600 umbrellas in May and 400 in June. Each umbrella sells for \($15\). Rainwaters beginning and ending finished goods inventories for May are 75 and 50 units,...
-
Don Moon is the owner of ABC Cleaning. At the beginning of the year, Moon had \(\$ 2,400\) in inventory. During the year, Moon purchased inventory that cost \(\$ 13,000\). At the end of the year,...
-
Agua Ole is a distributor of bottled water. For each of items a through c, compute the amount of cash receipts or payments Agua Ol will budget for September. The solution to one item may depend on...
-
If Mr. Jacobson chooses not to volunteer the information, could his hotel possibly face any liability in the future if the Wyandotte Hotel hires Lindsey and later learns the truth? Explain. lop5
-
Establish identity. cos( + k) = (-1)k cos , k any integer
-
Write the formula for the conjugate acid of each base. a. NH3 b. CIO C. HSO4 2- d. CO3-
-
As noted in the "A Word about . . . Methane, Marsh Gas, and Miller's Experiment" on page 60, methane can be formed in muddy sediments because of the reducing environment (i.e., lack of oxygen). Write...
-
Explain why 1,3-difluorobutane is a correct IUPAC name, but 1,3-dimethylpentane is not a correct IUPAC name.
-
Draw Newman projections for two different staggered conformations of butane (looking end-on at the bond between carbon-2 and carbon-3), and predict which of the two conformations is more stable. (If...
-
Jeannie is an adjunct faculty at a local college, where she earned $680.00 during the most recent semimonthly pay period. Her prior year-to-date pay is $18,540. She is single and has one withholding...
-
The company sold merchandise to a customer on March 31, 2020, for $100,000. The customer paid with a promissory note that has a term of 18 months and an annual interest rate of 9%. The companys...
-
imer 2 0 2 4 Question 8 , PF 8 - 3 5 A ( similar to ) HW Score: 0 % , 0 of 1 0 0 points lework CH 8 Part 1 of 6 Points: 0 of 1 5 Save The comparative financial statements of Highland Cosmetic Supply...

Study smarter with the SolutionInn App


