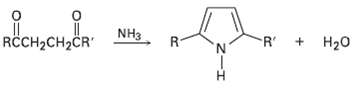
Substituted pyrroles are often prepared by treatment of a 1, 4-diketone with ammonia. Propose amechanism. R +
Question:
Substituted pyrroles are often prepared by treatment of a 1, 4-diketone with ammonia. Propose amechanism.
Transcribed Image Text:
R + H20 RCCH2CH2ČR" R- NH3 R.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (18 reviews)
HCCH 1 RC CR 11 O HCCH 11 1 RC HA HA 0NH proton N atio...View the full answer

Answered By

Sadoc Raju
I have been teaching High School Physics in India for the last nine years of my life.
I have taught in State Board, CBSE, ICSE and IGCSE schools.
I have gained incredible experience and valuable insight over these years having worked in five different schools in two states with a diverse school population.
I have been certified by Pune University, considered to be the premier institute for education in India.
I also have worked as an aircraft technician to gain practical knowledge in mechanics and aerospace.
Why do I teach?
For me, the rewards and challenges of teaching offer a sense of satisfaction that is enhanced by the knowledge that I have had some impact on the academic and personal development of the children I teach. I believe in developing with the children a supportive classroom environment, which has a basis in quickly developing rapport with the children. I pride myself to be a dedicated self-motivated achiever who is committed to success and adapt at juggling multiple tasks in a high-pressured environment.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Ammonia is a principal nitrogen fertilizer. It is prepared by the reaction between hydrogen and nitrogen. In a particular reaction, 6.0 moles of NH3 were produced. How many moles of H2 and how many...
-
Ammonia is compressed from 120 kPa with x = 1 to a pressure of 1.2 MPa and a temperature of 100C. For a mass flux of 3 kg/s, determine the power required to drive the adiabatic compressor.
-
A piston/cylinder contains 1 kg of ammonia at 20C with a volume of 0.1 m3, shown in Fig. P5.129. Initially the piston rests on some stops with the top surface open to the atmosphere, Po, so a...
-
A fast-food chain randomly attaches coupons for prizes to the packages used to serve french fries. Most of the coupons say Play again, but a few are winners. Seventy-five percent of the coupons pay...
-
In what ways does Costco meet the criteria for a "sustainable" organization?
-
FIGURE Q33.3 is the interference pattern seen on a viewing screen behind 2 slits. Suppose the 2 slits were replaced by 20 slits having the same spacing d between adjacent slits. a. Would the number...
-
How might Victor assess the extent to which this career move will help him to become a large-property general manager with his organization? How important should this long-term career concern be in...
-
Define the concept of scalability. Explain why it might be a good idea for owners of small businessesand managers in larger businessesto understand this concept.
-
The Common Stock account for Baltimore Corporation on January 1, 2020 was $60,000. On July 1, 2020 Baltimore issued an additional 10,000 shares of common stock. The Common Stock is $5 par. There was...
-
Question Mr. Woods, an electrician for Timberland city, has made some faulty connections on eight street lights. The errors cause a street light to go OFF if the street lights adjacent to that light...
-
Protonation of an amide using strong acid occurs on oxygen rather than on nitrogen. Suggest a reason for this behavior, taking resonance intoaccount. :0: H2SO4 NH2 NH2 R.
-
3, 5-Dimcthylisoxazole is prepared by reaction of 2, 4-pentanedionc with hydroxylamine. Propose amechanism. CH CH3CCH2CCH3 + H2NOH 3,5-Dimethylisoxazole
-
What are the sacrifices customers have to make in order to have access to this shopping experience?lop4
-
Consider the project information in the table below: Draw and analyze a project network diagram to answer the following questions: a. If you were to start on this project, which are the activities...
-
Lacey, Inc., had the following sales and purchase transactions during 2011. Beginning inventory consisted of 80 items at \(\$ 120\) each. Lacey uses the FIFO cost flow assumption and keeps perpetual...
-
Refer to the Camp Sunshine data presented in E5-9. Required: 1. Perform a least-squares regression analysis on Camp Sunshines data. 2. Using the regression output, create a cost equation (Y = A + BX)...
-
The following information pertains to the first year of operation for Sonic Boom Radios, Inc. Required: Prepare Sonic Booms full absorption costing income statement and variable costing income...
-
Jane Crawford, the president of Crawford Enterprises, is considering two investment opportunities. Because of limited resources, she will be able to invest in only one of them. Project A is to...
-
If Mr. Jacobson decides to tell Erica Stovall that Lindsey was terminated for theft, what might be the legal ramifications, if any, for the Argos Hotel? Explain your answer. lop5
-
Use the information given about the angles and to find the exact value of: (a) sin( + ) (b) cos( + ) (c) sin( - ) (d) tan ( + ) (e) sin(2) (f) cos (2) (g) sin /2 (h) cos/2 cos = 4/5, 0 < < /2; cos =...
-
Write the formula for the conjugate base of each acid. a. HCI b. HSO3 c. HCHO, d. HF
-
What are all of the structural possibilities for C4H6? (Nine compounds, four acyclic and five cyclic, are known.)
-
Write an equation for each of the following reactions: a. 2-butene + HCl b. 3-hexene + HI c. 4-methylcyclopentene + HBr
-
Use Markovnikov's Rule to predict which regioisomer predominates in each of the following reactions: a. 1-pentene + HBr b. 2-methyl-2-hexene + H2O (H+ catalyst)
-
As a Financial Analyst in the Finance Department of Zeta Auto Corporation they are seeking to expand production. The CFO asks you to help decide whether the firm should set up a new plant to...
-
Chapter 4 When an Auditor finds misstatements in entities financial statements which may be the result of fraudulent act, what should be the role of an auditor under that situation? (2 Points)
-
Suppose the following input prices are provided for each year: Required: $

Study smarter with the SolutionInn App


