Histamine, whose release in the body triggers nasal secretions and constricted airways, has three nitrogen atoms. List
Question:
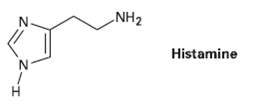
Histamine, whose release in the body triggers nasal secretions and constricted airways, has three nitrogen atoms. List them in order of increasing basicity, and explain yourordering.
Transcribed Image Text:
NH2 Histamine H.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (15 reviews)
b NH N bra CN Histamine 1 H The a nitrogen is mo...View the full answer

Answered By

Shikhar Srivastava
I have interest in science as it enables us to understand the process happening in our universe. I am a subject matter expert at Chegg solving advance physics problem with CF score more than 80% on average. I like to help student by solving problems. This helps me as well to build up my concepts regarding the subject. I have learnt from my experience that, In physics to build up command on subject one need to understand the theory, keep revising notes in free time and at last one more important thing is that one need to keep solving numerical problem related to the topic.
Taking career in core sciences is a long journey and one need to be calm minded with his or her profession.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Explain the basicity order of the following three amines: p-nitroaniline (A), rz-nitroaniline (A), and aniline (C). The structures and pKa data are shown in Table 23'l'
-
The nitrogen atoms in N2 participate in multiple bonding, whereas those in hydrazine, N2H4, do not. (a) Draw Lewis structures for both molecules. (b) What is the hybridization of the nitrogen atoms...
-
List the three states of matter in order of] (a) Increasing molecular disorder and (b) Increasing intermolecular attractions. (c) Which state of matter is most easily compressed?
-
Doug Brackett wants to have enough mechanics on hand to take care of his customer requests, but he does not want to be paying mechanics to sit around doing nothing. Doug needs to know a reasonable...
-
Based on the description in this case, how well would you say Susan Durbin appreciates the scope of human resource management? What, if any, additional skills of an HR professional would you...
-
Suppose you have two pinhole cameras. The first has a small round hole in the front. The second is identical except it has a square hole of the same area as the round hole in the first camera. Would...
-
How should future training of expatriates be modified based on Charless experiences?
-
The medical director of a large emergency clinic faces a problem of providing treatment for patients who arrive at different rates during the day. There are four doctors available to treat patients...
-
PETRON Company issued 7 5 , 0 0 0 new shares of its P 5 par value ordinary shares valued at P 1 2 per share in exchange for 9 0 % of outstanding shares of SHELL Company on March 3 1 , 2 0 2 2 . The...
-
The proposed rates were not in the range the CEO expected given the pricing analysis. The CEO has asked the pricing actuary to verify the total projected loss cost excluding potential large storm...
-
Although pyrrole is a much weaker base than most other amines, it is a much stronger acid (pK a 15 for the pyrrole versus 35 for diethyl amine). The NH proton is readily abstracted by base to yield...
-
Oxazole is a live-membered aromatic heterocycle. Would you expect oxazole to be more basic or less basic than pyrrole?Explain. Oxazole N:
-
What are the practical consequences of a lack of strategic linkage between the business and the operations function?
-
When a supersonic airflow, \(M=1.8\), passes through a normal shockwave under sea level conditions, what are the values of the stagnation pressure before and after the normal shockwave?
-
Eastern University, located in central Canada, prides itself on providing faculty and staff with a competitive compensation package. One aspect of this package is a tuition benefit of \($4,000\) per...
-
What is the formula for calculating return on investment (ROI)?
-
Air enters a 5.5-cm-diameter adiabatic duct with inlet conditions of \(\mathrm{Ma}_{1}=2.2, T_{1}=250 \mathrm{~K}\), and \(P_{1}=60 \mathrm{kPa}\), and exits at a Mach number of...
-
At the various activity levels shown, Taylor Company incurred the following costs. Required: Identify each of these costs as fixed, variable, or mixed. Units sold 20 40 60 80 100 a. Total salary cost...
-
Conduct a general Internet search and locate two or three companies in the hospitality industry business that provide preemployment screening. What kinds of services do the companies offer? If you...
-
Explain how the graph of each function can be obtained from the graph of y = 1/x or y = 1/x 2 . Then graph f and give the (a) Domain (b) Range. Determine the largest open intervals of the domain over...
-
Classify each acid as strong or weak. If the acid is weak, write an expression for the acid ionization constant (K a ). a. HNO3 b. HCI c. HBr d. HSO3
-
Using structural formulas, write equations for each of the following combustion reactions For information: (see Reaction Summary 1.a ): a. The complete combustion of propane b. The complete...
-
Write the formula for each of the following compounds: a. Isobutyl chloride b. isopropyl bromide c. 2-chlorobutane d. tert-butyl iodide e. Propyl fluoride f. general formula for an alkyl bromide
-
Using structural formulas, write equations for the following halogenation reactions (see Reaction Summary 1.b, p. 63), and name each organic product: a. The monochlorination of propane b. The...
-
The following are the information of Chun Equipment Company for Year 2 . ( Hint: Some of the items will not appear on either statement, and ending retained earnings must be calculated. ) Salaries...
-
Alta Ski Company's inventory records contained the following information regarding its latest ski model. The company uses a periodic inventory system. Beginning inventory, January 1, 2018 1,250 units...
-
Fibertech GmbH is a distributor of outdoors technical clothing. The company outsources the production of clothing to external manufacturers in Bangladesh and sells the clothing under its own brands....

Study smarter with the SolutionInn App


