Explain which route would provide a better synthesis of theseethers: CH3 CH, CH3 CHI + CH,CO a)
Question:
Explain which route would provide a better synthesis of theseethers:
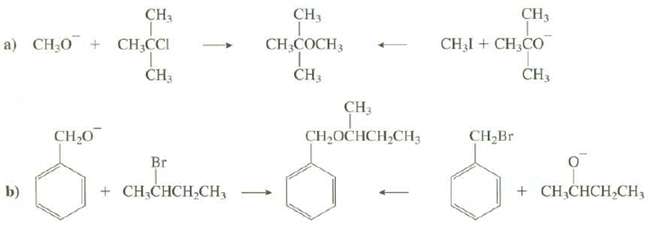
Transcribed Image Text:
CH3 CH, CH3 CHẠI + CH,CO a) CH.O + CH,CCI CH3 CH,COCH3 ČH3 CH3 CH, CH,OCHCH,CH; CH,Br CH,0 Br + CH;CHCH,CH3 b) + CH;CHCH,CH,
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
a The right route is better because methyl iodide cannot undergo an e...View the full answer

Answered By

Bree Normandin
Success in writing necessitates a commitment to grammatical excellence, a profound knack to pursue information, and a staunch adherence to deadlines, and the requirements of the individual publication. My background comprises writing research projects, research meta-analyses, literature reviews, white paper reports, multimedia projects, reports for peer-reviewed journals, among others. I work efficiently, with ease and deliver high-quality outputs within the stipulated deadline. I am proficient in APA, MLA, and Harvard referencing styles. I have good taste in writing and reading. I understand that this is a long standing and coupled with excellent research skills, analysis, well-articulated expressions, teamwork, availability all summed up by patience and passion. I put primacy on client satisfaction to gain loyalty, and trust for future projects. As a detail-oriented researcher with extensive experience surpassing eight years crafting high-quality custom written essays and numerous academic publications, I am confident that I could considerably exceed your expectations for the role of a freelance academic writer.
5.00+
7+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Explain which of the reaction would provide a better synthesis of3-hexanone. . . CH,CH,CH,CH,CH, 3-Hexanone CH,CH,C=CCH CH3 CH,C=CCH,CH,CH3 H,SO4 HgSO, H&SO,
-
Explain which of these reactions would provide a better synthesis of2-pentene: Br CH,OH CH,CH,CHCH,CH, + CH;0 CH,CH=CHCH,CH3 Br CH, CH CH=CHCH CH, CH,CHCH,CH,CH; + CH,0
-
Provide a retrosynthetic analysis and synthesis for each of the following compounds. Permitted starting materials are phenylmagnesium bromide, oxirane, formaldehyde, and alcohols or esters of four...
-
Evaluate the integral (4e* + 2 In (2))dx.
-
Some businesses use only approximations when estimating cost functions. What are some possible reasons for this?
-
The following annual account balances are taken from Armour Sports at December 31. ______________________________________ 2017 _____________ 2016 Accounts receivable .......................$ 100,000...
-
Review the templates for various project documents provided in this chapter. Pick one of them and apply it to a project of your choice. Make suggestions for improving the template. LO.1
-
Presented here are the accounts of Town and Country Realty for the year ended December 31, 2016: Land............ $ 5,000 Notes Payable......... 36,000 Property Tax Expense...... 3,400...
-
Scenario: For the past several years, Angela Smith operated a part-time consulting business from her home. As of September 1, 2020, Angela decided to move to rented quarters and to operate the...
-
Moe's Landscaping company offers lawn maintenance services. Two frequent transactions for the company are billing customers for services performed and paying employee salaries. For example, on March...
-
Diphenhydramine can also be synthesized by heating bromo diphenyl methane with the amino alcohol shown here. Offer a reason why the oxygen, rather than the nitrogen, of this compound acts as the...
-
Suggest a synthesis of these ethers starting with an alcohol and an alkylhalide: OCH CH,CH a) CH,OCH,CH,CH,CH, b) c)
-
In Exercises 1 through 28, differentiate the given function. 7 1.2 X + in -2.1 X
-
For the past 30 years, the average satisfaction rating for a sushi restaurant has been 3.9 out of 5. If the rating for a sample of 256 people is 4.1 with a standard deviation of 0.5, the critical...
-
Hash collisions occur when more than one item is mapped to the same element in Hash Table's array. What is one way that a Hash Table can handle collisions?
-
Scatterplot. In Exercises 5-8, use the sample data to construct a scatterplot. Use the first variable for the x-axis. Based on the scatterplot, what do you conclude about a linear correlation? Pulse...
-
Given two fair six sided dice and a standard deck of 52 playing cards, calculate the probability of a rolling a sum of 7 or 11 and drawing three cards in which at least one is a face card.
-
z Scores. In Exercises 5-8, express all z scores with two decimal places. 5. Diastolic Blood Pressure of Females For the diastolic blood pressure measurements of females listed in Data Set 1 "Body...
-
What potential pitfalls need to be guarded against when devising coding schedules and manuals?
-
The text defined intrinsic value as the value of an asset given a hypothetically complete understanding of the assets investment characteristics. Discuss why hypothetically is included in the...
-
Solve each problem. 290% of 137 miles is what?
-
Rank the following dienophiles in order of their expected reactivity in the Diels?Alder reaction. CH CH NC NC CN c=C CH c=c c=C c=C NC CN
-
1, 3-Cyclopentadiene is very reactive in DielsAlder cyclo addition reactions, but 1, 3-cyclohexadiene is less reactive and 1, 3-cycloheptadiene is nearly inert. Explain. (Molecular models are...
-
1, 3-Pentadiene is much more reactive in Diels?Alder reactions than 2, 4-pentadienal. Why might this be? H 1,3-Pentadiene 2,4-Pentadienal
-
1. (A nice inharitage) Suppose $1 were invested in 1776 at 3.3% interest compounded yearly a) Approximatelly how much would that investment be worth today: $1,000, $10,000, $100,000, or $1,000,000?...
-
Why Should not the government subsidize home buyers who make less than $120K per year. please explain this statement
-
Entries for equity investments: 20%50% ownership On January 6, 20Y8, Bulldog Co. purchased 25% of the outstanding common stock of $159,000. Gator Co. paid total dividends of $20,700 to all...

Study smarter with the SolutionInn App


