Addition of HBr to 1-phenyipropene yields only (1-bromopropyl) benzene. Propose a mechanism for the reaction, and explain
Question:
Addition of HBr to 1-phenyipropene yields only (1-bromopropyl) benzene. Propose a mechanism for the reaction, and explain why none of the other regioisomer isproduced.
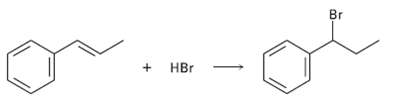
Transcribed Image Text:
Br + HBr
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (13 reviews)
HBr o Protonation of the double bond ...View the full answer

Answered By

Joseph Mwaura
I have been teaching college students in various subjects for 9 years now. Besides, I have been tutoring online with several tutoring companies from 2010 to date. The 9 years of experience as a tutor has enabled me to develop multiple tutoring skills and see thousands of students excel in their education and in life after school which gives me much pleasure. I have assisted students in essay writing and in doing academic research and this has helped me be well versed with the various writing styles such as APA, MLA, Chicago/ Turabian, Harvard. I am always ready to handle work at any hour and in any way as students specify. In my tutoring journey, excellence has always been my guiding standard.
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Propose a mechanism for the reaction of acetyl chloride with phenylmagnesium bromide to give 1,1-diphenylethanol. OH (1) ether solvent CH3-C CI 2 (2) H 0 acetyl chloride phenylmagnesium bromide...
-
Propose a mechanism for the reaction of cyclohexyl methyl ketone with excess bromine in the presence of sodium hydroxide.
-
Propose a mechanism for the reaction of an amide with thionyl chloride to form a nitrile.
-
How did your parents communication with you influence your self-concept?
-
Sam, age 45, is single. For 2016, he has the following items: Business income........................................................$70,000 Business...
-
The following data show the retail price for 12 randomly selected laptop computers along with their corresponding processor speeds in gigahertz. a. Develop a linear equation that can be used to...
-
The amount of interest revenue accrued at December 31 (the companys year-end) is: a. $1,500 b. $1,375 c. $1,000 d. $625 e. $300 AppendixLO1
-
As the auditor of Clearwater County you learn that various assets are subject to spending constraints. Indicate how each of the following constraints would affect the countys reported fund balance...
-
la 19 0/15 F Company receives $600 from a customer on April 1. In return F Company will provide security services for 6 months starting in April. F will provide the same amount of services each...
-
Refer to the real estate investment situation in Problem 10-17. (a) Suppose that the shopping center and the apartment would be on adjacent properties, and the shopping center would only be...
-
Electrophilic substitution on 3-phenylpropanenitrile occurs at the ortho and Para positions, but reaction with 3-phenylpropenenitrile occurs at the meta position. Explain using resonance structures...
-
Triphenylmethane can be prepared by reaction of benzene and chloroform in the presence of A1Cl3. Propose a mechanism for thereaction. H. + CHCI3 AICI3
-
A vapour-compression refrigeration cycle in which the refrigerant HFC-134a enters the compressor as superheated vapour at \(0.18 \mathrm{MPa}\) and \(-10^{\circ} \mathrm{C}\) at a rate of \(0.06...
-
The adjusted trial balance section of Menlo Company's worksheet shows a \(\$ 1,500\) debit balance in utility expense. At the end of the accounting period the accounting manager accrues an additional...
-
Identify each of the 10 amount columns of the worksheet and indicate to which column the adjusted balance of the following accounts would be extended: a. Accounts Receivable b. Accumulated...
-
Using the data from Table 3.3, show the effect on world output if each country moved toward specialization in the production of its comparative-disadvantage good. TABLE 3.3 Comparative Advantage as a...
-
The Professional Winner was RJ Andrews from Info We Trust, for the video Are Gazelles Endangered? (a) Watch this video. What data are this video conveying? (b) You can interact with the data and...
-
(a) Draw a simplified ray diagram showing the three principal rays for an object located outside the focal length of a converging lens. (b) Is the image real or virtual? (c) Is it upright or...
-
What do you think has contributed to SASs low turnover? Why is low turnover good for a company?
-
Identify Thank You mission, strategy and core competencies. Identify strategy changes that have taken place at Thank You since its founding in 2008. Your answer must in text references and must be...
-
Derive the following Maxwell relations for open systems. a. Starting from Eq. 6.2-5a, b. Starting from Eq. 6.2-6a, c. Starting from Eq. 6.2-7a, d. Starting from Eq. 6.2-8a, (). HT ON (0) aN S, N S,V...
-
Show the products of thesereactions: CH NaOH Br2 Bra b) a) . .
-
The reaction of an alkenes with bromine in an alcohol as solvent produces as ether as the product. Show a mechanism for the following reaction and explain the stereochemistry of theproduct. Br . H....
-
Show all the steps in the mechanism for the formation of MTBE from methanol and isobutylene.
-
Product Weight Sales Additional Processing Costs P 300,000 lbs. $ 245,000 $ 200,000 Q 100,000 lbs. 30,000 -0- R 100,000 lbs. 175,000 100,000 If joint costs are allocated based on relative weight of...
-
The projected benefit obligation was $380 million at the beginning of the year. Service cost for the year was $21 million. At the end of the year, pension benefits paid by the trustee were $17...
-
CVP Modeling project The purpose of this project is to give you experience creating a multiproduct profitability analysis that can be used to determine the effects of changing business conditions on...

Study smarter with the SolutionInn App


