Question: At what temperature will the diffusion coefficient for the diffusion of copper in nickel have a value of 6.5 ? 10 -17 m 2 /s.
At what temperature will the diffusion coefficient for the diffusion of copper in nickel have a value of 6.5 ? 10-17 m2/s. Use the diffusion data in Table 5.2.
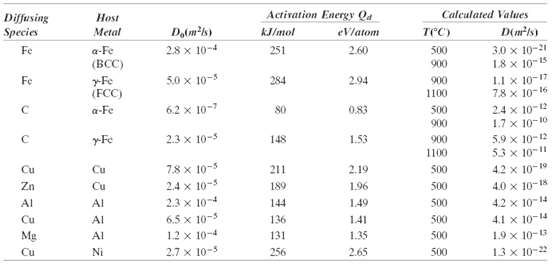
Diffusing Species Host Activation Energy Qa Calculated Values Metal kJ/mol eVlatom TCC) D(mls) Fe a-Fe (BCC) 28 x 10-4 251 2.60 500 3.0 x 10-21 18 x 10-15 L1 x 10-17 78 x 10-16 24 x 10-12 1.7 x 10-10 5.9 x 10-12 53 x 10-11 4.2 x 10-19 900 Fe rFe (FCC) 5.0 x 10-5 284 2.94 900 1100 a-Fe 6.2 x 10-7 80 0.83 500 900 y-Fe 2.3 x 10-5 148 1.53 900 1100 Cu Cu 7.8 x 10-5 211 2.19 500 2.4 x 10-5 2.3 x 10-4 Zn Cu 189 1.96 500 4.0 x 10-18 Al Al 144 1.49 500 4.2 x 10-14 Cu Al 6.5 x 10-5 136 141 500 4.1 x 10-14 1.9 x 10-13 Mg Al 12 x 10-4 131 1.35 500 Cu Ni 2.7 x 10-5 256 2.65 500 13 x 10-2
Step by Step Solution
3.29 Rating (170 Votes )
There are 3 Steps involved in it
Solving for T from Equation 59a and using the data from Table 52 for the diffusion of ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (143).docx
120 KBs Word File


