Bond strengths can be used to estimate the relative stability of isomers that have different bonds. The
Question:
Bond strengths can be used to estimate the relative stability of isomers that have different bonds. The isomer that has the larger total bond energy is more stable. One of the following isomers is more stable than the other. The less stable one is rapidly converted Co the more stable one, so it cannot be isolated. On the basis of bond dissociation energies, which of these two isomers is more stable?
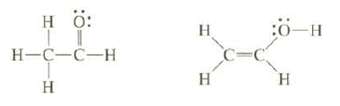
Transcribed Image Text:
H ö: H-C-C-H Т Н н H :ӧ-н H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (22 reviews)
Use Table 21 in the text to calculate total bond energies of compound...View the full answer

Answered By

Sarah Khan
My core expertise are:
-_ Finance
-_ Business
-_ Management
-_ Marketing Management
-_ Financial Management
-_ Corporate Finance
-_ HRM etc...
I have 7+ years of experience as an online tutor. I have hands-on experience in handling:
-_ Academic Papers
-_ Research Paper
-_ Dissertation Paper
-_ Case study analysis
-_ Research Proposals
-_ Business Plan
-_ Complexed financial calculations in excel
-_ Home Work Assistance
-_ PPT
-_ Thesis Paper
-_ Capstone Papers
-_ Essay Writing etc...
5.00+
91+ Reviews
92+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The land-pan-evaporation formula may be used to estimate the evaporation rate from swimming pools. Compare the water loss rate for a pool in Pasadena, California, exposed to air at 80F and 30 percent...
-
What methods can be used to estimate the energy requirements of a job?
-
The formula t = d/4 can be used to estimate the time, t, in seconds it takes for an object dropped to travel d feet. (a) Estimate the time it takes for an object to drop 100 ft. (b) Estimate the time...
-
Governments assert that their safety standards for food imports are important to ensure that their citizens not be harmed by unsafe foods. Comment on how such a concern may be a nontariff barrier.
-
Zap is a retailer that specialises in electrical goods. It is a division of a large retail company, data relate to the most recent year of operations:...
-
During 2017, Nilsen Company started a construction job with a contract price of $1,600,000. The job was completed in 2019. The following information is available. The contract is non-cancellable....
-
To construct a p-chart, 20 samples of size 150 were drawn from a process. The proportion of defective items found in each of the samples is listed in the next table. Sample Proportion Defective...
-
Dawayne Wade Company purchased equipment for $212,000 on October 1, 2008. It is estimated that the equipment will have a useful life of 8 years and a salvage value of $12,000. Estimated production is...
-
Responsibilitys of the patient safety attendant (PSA) include which of the following? Najs
-
Jimmy owns a garden in which he has planted N trees in a row. After a few years, the trees have grown up and now they have different heights. Jimmy pays much attention to the aesthetics of his...
-
Because of two hydrogen bonds, carboxylic acids show a very strong attractive force between two molecules that persists even in the gas phase. Show this hydrogen bonding between two carboxylic acid...
-
Bond strengths can be used to estimate the relative stability of isomers that have different bonds. The isomer that has the larger total bond energy is more stable. One of the following isomers is...
-
Research the case Google Spain v. AEPD and Mario Costeja Gonzalez. This case pits a particular kind of privacythe right for certain information not to show up in a search resultagainst the right to...
-
The table 1 below is shown the production theory of labour for company D'LIMAU Sdn Bhd. Input X Input Y Total Product (TP/Q) 1 0 0 1 1 20 1 2 80 1 3 180 1 4 230 1 5 270 1 6 270 1 7 210 Average...
-
Describe the most important three rights in the Bill of Rights of the United States Constitution. The Bill of Rights is the first ten amendments to the Constitution, but they contain far more than...
-
A red/white wine-tasting party will be held in the school in April, and the theme of the event will be formulated based on the season (April), place (Ontario/Canada), and target participants. Please...
-
We explored many of the revenue streams available to fund fire prevention practices. Describe one revenue stream and explain why you feel it is the most important in supporting fire prevention...
-
Multi-national management in a global economy requires a variety of hard and soft skills. This assignment is meant to enhance the understanding of multi-national situations locally or globally, and...
-
Have you ever made a personal goal public in the hope that it would help you accomplish it? Explain.
-
Havel says the grocer doesnt believe what is on the sign and indeed, he says the grocers customers will barely notice it. But Havel maintains that the sign serves a specific function. How would you...
-
If f(x) = x 2 and g(x) = x 3, find the composite functions f g and g f.
-
The herbicide acifluorfen can be prepared by a route that begins with reaction between a phenol and an aryl fluoride. Propose a mechanism. NO2 CO2CH3 NO2 co,CH3 NO2 .CO2H DMSO HO F3C F3C CI FC...
-
The red fox (Vulpes) uses a chemical communication system based on scent marks in urine. Recent work has shown one component of fox urine to be a sulfide. Mass spectral analysis of the pure...
-
Anethole, C10H12O, a major constituent of the oil of anise, has the 1H NMR spectrum shown. On oxidation with Na2Cr2O7, Anethole yields p-methoxybenzoic acid. What is the structure of Anethole? Assign...
-
Based on the regression output (below), would you purchase this actively managed fund with a fee of 45bps ? Answer yes or no and one sentence to explain why.
-
What is the yield to maturity on a 10-year, 9% annual coupon, $1,000 par value bond that sells for $967.00? That sells for $1,206.10?
-
1)Prepare the journal entry to record Tamas Companys issuance of 6,500 shares of $100 par value, 9% cumulative preferred stock for $105 cash per share. 2. Assuming the facts in part 1, if Tamas...

Study smarter with the SolutionInn App


