Calculate formal charges for the non-hydrogen atoms in the following molecules: (a) Diazomethane, (b) Acetonitrile oxide, (c)
Question:
Calculate formal charges for the non-hydrogen atoms in the following molecules:
(a) Diazomethane,
(b) Acetonitrile oxide,
(c) Methylisocyanide,
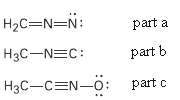
Transcribed Image Text:
H2C=N=N: part a H3C-NEC: part b H3C-CEN-o: part c
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (8 reviews)
Strategy To find the formal charge of an atom in a molecule follow these two steps 1 Draw an e...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Identify the carbon atoms in the following molecules as primary, secondary, tertiary, orquaternary: H H CH CH CHCH2CHCH2CH Gs (c) (a) (b) CH2H2CH CHCCH2CCH CH
-
A few hydrogen and oxygen molecules are introduced into a container in the quantities depicted in the following drawing. The gases are then ignited by a spark, causing them to react and form H2O. a....
-
In the following four models, C atoms are black, H atoms are light blue, O atoms are red, and N atoms are dark blue: a. Write the molecular formula of each molecule. b. Write the condensed structural...
-
True Or False Death benefits are used to compensate the deceaseds family for pain and suffering.
-
Near the end of 2016, Byron realizes that he has a net short-term capital loss of $13,000 for the year. Byron has taxable income (not including the loss) of $123,000 and is single. He owns numerous...
-
1. Do you agree that corporations derive benefits from sponsoring volunteer programs and encouraging employees to participate? 2. The CEO of Hands on Miami write a letter persuading Miami...
-
Using the PewSocialMedia dataset, get a 95% confidence on the recoded variable Number of Texts Sent Per Day (XYtextnum1) for two groups: whites and non-whites. Present your confidence intervals, and...
-
A taxpayer is about to receive a $ 1,000 bonus payment from his employer. He would like to put this bonus into a retirement account. He has come to you for advice as to whether he should put the $...
-
Which of the following is not a coherence strategy? Select one: O a. Starting with a general idea and then gradually shifting to more specific ideas b. Expressing one view and then switching to an...
-
Vista City hospital plans the short-stay assignment of surplus beds (those that are not already occupied) 4 days in advance. During the 4-day planning period about 30, 25, and 20 patients will...
-
Nitromethane has the structure indicated. Explain why it must have formal charges on N andO. :0: Nitromethane :O:
-
Organic phosphate groups occur commonly in biological molecules. Calculate formal charges on the four O atoms in the methyl phosphatedianion. 2- :0: H-C-0-P-0: Methyl phosphate :0:
-
Many companies make annual reports available on their corporate Internet home page. Annual reports also can be accessed through the SECs EDGAR system at www.sec.gov (under Filing Type, search for...
-
Based on the following information, calculate the sustainable growth rate for Kaleb's Welding Supply: Profit margin Capital intensity ratio Debt-equity ratio Net income Dividends 7.5% 0.65 0.60...
-
Waterway Inc. uses LIFO inventory costing. At January 1, 2025, inventory was $216,014 at both cost and market value. At December 31, 2025, the inventory was $283,252 at cost and $262,660 at market...
-
What is the 32-bit version of: 0000 0000 0001 0101
-
1. Let A = 2 1 4 3 Find AT, A-1, (A-1) and (AT)-1. 2. Let A = = [ -1 -1 2 22 (a) Find (AB), BT AT and AT BT. (b) (AB)-1, B-1A-1 and A-B-1. ] 1-5 and B = 1
-
Xavier Ltd. paid out cash dividends at the end of each year as follows: Year Dividend Paid 2018 $250,000 2019 $325,000 2020 $400,000 Assume that Xavier had 100,000 common shares and 5,000, $4,...
-
Which statement is most appropriate? a. There are differing views of IS audit with many believing that all audit sections should employ specialist auditors. Others feel there is no such animal as the...
-
What is the difference between the straight-line method of depreciation and the written down value method? Which method is more appropriate for reporting earnings?
-
a. Quantitatively explain why ice skates slide along the surface of ice. Can it get too cold to ice skate? b. Is it possible to ice skate on other materials, such as frozen carbon dioxide? c. What is...
-
Explain why the pKas of compounds near the middle of Table 4.2 are often listed with two figures to the right of the decimal place (that is, for NH4+ the pKa = 9.24), whereas those at the beginning...
-
For each pair of compounds, explain which is the stronger acid?
-
Explain why the compound on the left is a stronger acid than the compound on the right.
-
business law A partner may actively compete with the partnership True False
-
A company provided the following data: Selling price per unit $80 Variable cost per unit $45 Total fixed costs $490,000 How many units must be sold to earn a profit of $122,500?
-
Suppose a 10-year, 10%, semiannual coupon bond with a par value of $1,000 is currently selling for $1,365.20, producing a nominal yield to maturity of 7.5%. However, it can be called after 4 years...

Study smarter with the SolutionInn App


