Organic phosphate groups occur commonly in biological molecules. Calculate formal charges on the four O atoms in
Question:
Organic phosphate groups occur commonly in biological molecules. Calculate formal charges on the four O atoms in the methyl phosphatedianion.
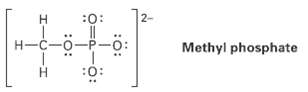
Transcribed Image Text:
2- :0: H-C-0-P-0: Methyl phosphate :0:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
Formal charge FC of bonding electrons neering of v...View the full answer

Answered By

Joemar Canciller
I teach mathematics to students because I love to share what I have in this field.
I also want to see the students to love math and be fearless in this field.
I've been tutoring these past 2 years and I would like to continue what I've been doing.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Calculate the formal charges on all of the atoms except hydrogen's, in these compounds: a) H-N-N=N: c) H-C-N=N: H e) H H-=C-H b) H-N-N-N: HO: H-C-C-C 1 H d) f) H H-B-H T H
-
Calculate the formal charges on the atoms shown inred. (a) (CH3)2OBF3 (b) H2C-NEN: (c) H2C=N=N: (d) :=-: (e) H (f) - CH
-
Calculate formal charges for the non-hydrogen atoms in the following molecules: (a) Diazomethane, (b) Acetonitrile oxide, (c) Methylisocyanide, H2C=N=N: part a H3C-NEC: part b H3C-CEN-o: part c
-
Discuss the primary sources of nonverbal communication.
-
In general, the 45-day identification period and the 180-day exchange period for like-kind exchanges cannot be extended. Does this rule change if the like-kind property or the taxpayer involved in...
-
What employment trends are occurring in todays workplace?
-
Using the PewSocialMedia dataset, run a frequency distribution that addresses this question: among those aged 35 and younger, what percentage of the sample uses Facebook? Then build a 99% confidence...
-
Using the information in E22-3, assume that in July 2014, Thome Company incurs The following manufacturing overhead costs. Instructions (a) Prepare a flexible budget performance report, assuming that...
-
Which of the following, if placed into service in 2021, is listed property? A. A passenger car B. An office chair C. A retail store building D. A cell phone Test-Annal Federal Tax Ratrasite ection...
-
A sports apparel company has received an order for a college basketball teams national championship T-shirt. The company can purchase the T-shirts from textile factories in Mexico, Puerto Rico, and...
-
Nitromethane has the structure indicated. Explain why it must have formal charges on N andO. :0: Nitromethane :O:
-
Draw the indicated number of resonance forms for each of the following species: (a) The methyl phosphate anion, CH3OPO32- (3) (b) The nitrate anion, NO3- (3) (c) The allyl cation, H2C = CH ? CH2+ (2)...
-
Prior to reading this chapter, had you heard of the "glass escalator"? Are you aware of a situation in which a man is the manager or supervisor of a group of women employees?
-
Complete Exercises 2-B and 2-H in Writing and Analysis in the Law using what you learned in the reading and in the Seminar. Use paragraph form, use complete sentences, and make sure you use proper...
-
What is the value of a stock expected to be in 9 years if the annual dividend is expected to remain unchanged forever at $3.65, the expected rate of return is 6.9% per year, and the next dividend is...
-
Once invested IN a corporation, shareholders want their money out - they want a return on investment! John owns 2 5 % of REFUND CORP INC, which paid out a $ 5 0 , 0 0 0 distribution to him on 1 2 / 3...
-
Worksheet Financial Statement Ratios. Lowe's Companies, Inc Jan 28, 2022 and Jan. 29, 2021 Current Ratio Current Assets / Current Liabilities Acid Test Current Assets Current Liabilities (Cash + ST...
-
3. Peter Senen operates in a JIT manufacturing system. For August, Peter Senen purchased 10,000 units of raw materials at P1.00 per unit on account.What is the The journal entry to record the...
-
Which statement is least appropriate? There are several options for securing the necessary IS/IT skills for internal auditing: a. Use a consortium to provide the necessary skills. b. Use a small...
-
Place a tick in the appropriate grid to identify the balance that would be brought down in each of the following named accounts, in the books of Rizwy Mohamed: (a) In the Cash account: if Rizwy...
-
The effect of pressure on the melting temperature of solids depends on the heat of fusion and the volume change on melting. The heat of fusion is always positive (that is, heat must be added to melt...
-
Provide IUPAC name for these alkenes: CH3 a) CHCHCHCHCH CH3 c) CHCHCHCHCH3 e) CHCH3 CH3 b) CHCHCHCHCH CHCH3 d) CHCHCHCHCCHCHCHCH T CH, CHCH CH, f)
-
Draw the structures of these compounds: (a) 4-Methylocatane (b) 2, 4-Dimethyl-5 propyl decane
-
What is wrong with these names? Provide the correct name for each. (a) 5, 5 Dimethyl-3-ethylhexane (b) 2-Dimethyl pentane
-
44. Dryer Companys policy is to keep 25% of the next month's sales in ending inventory. If Dryer meets its ending inventory policy at the end of April and sales are expected to be 24,000 units in May...
-
What general conclusions can you draw about your companys liquidity, solvency and productivity based on your ratio calculations. Working Capital 2017 = $9,994 M 2016 = $10,673 M Current Ratio 2017 =...
-
Tami Tyler opened Tami's Creations, Incorporated, a small manufacturing company, at the beginning of the year. Getting the company through its first quarter of operations placed a considerable strain...

Study smarter with the SolutionInn App


