Calculate the pressure exerted by 0.5000 mole of N 2 in a 1.0000-L container at 25.0 o
Question:
Calculate the pressure exerted by 0.5000 mole of N2 in a 1.0000-L container at 25.0oC. (See Table)
a. Use the ideal gas law.
b. Use the van der Waals equation.
c. Compare the results from parts a and b.
Table
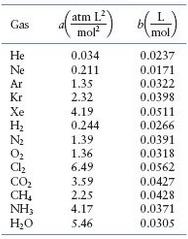
Transcribed Image Text:
\ 7 1 200 1 6 1 8 2 7815 2133 (000000000000 691622 23354433 LF- 11 5 2 9 4 9699576 345214 m | 0 2 3 3 1 2 3 0012401163245
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (10 reviews)
a b c PV nRT P n...View the full answer

Answered By

Bhartendu Goyal
Professional, Experienced, and Expert tutor who will provide speedy and to-the-point solutions. I have been teaching students for 5 years now in different subjects and it's truly been one of the most rewarding experiences of my life. I have also done one-to-one tutoring with 100+ students and help them achieve great subject knowledge. I have expertise in computer subjects like C++, C, Java, and Python programming and other computer Science related fields. Many of my student's parents message me that your lessons improved their children's grades and this is the best only thing you want as a tea...
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Physical Chemistry questions
-
Calculate the pressure exerted by 1.0 mol H2S behaving as (a) A perfect gas, (b) A van der Waals gas when it is confined under the following conditions: (i) At 273.15 K in 22.414 dm3, (ii) At 500 Kin...
-
Van der Waals Equation and Critical Points (a) In p V- diagrams the slope p/V along an isotherm is never positive. Explain why. (b) Regions where p/V = 0 represent equilibrium between two phases;...
-
By what magnitude would the pressure exerted by water on the walls of the vessel have increased if the intermolecular attraction forces had vanished?
-
Assume that your team has been in contract with the headquarters of a company that owns several restaurants in different states in the US. Your team is to provide software that manages these...
-
Allies Apples, Inc. purchases apples in bulk and sells two products, boxes of apples and jugs of cider. Allies has capacity limitations of three kinds: warehouse space, crating facilities, and...
-
Almost certainly you have seen vending machines being serviced on your campus and elsewhere. On a predetermined schedule, the vending company checks each machine and fills it with various products....
-
1. The village owns general fixed assets with a historical cost of $100,000 and accumulated depreciation totaling $65,000.
-
a. Make an exponential smoothing forecast for periods 2 through 5 with 2 values of alpha, 0.05 and 0.60, and an assumed forecast for period 1 of 30. b. Compute the MAD for each of the above...
-
Xavier company is going through Chapter 7 bankruptcy. All assets have been liquidated, and the company retains only $26,200 in free cash. The following debts, totaling $43,050, remain: Government...
-
The income statement for Pruitt Company summarized for a four-year period shows the following: An audit revealed that in determining these amounts, the ending inventory for 2017 was overstated by...
-
It took 4.5 minutes for 1.0 L of helium to effuse through a porous barrier. How long will it take for 1.0 L of Cl 2 gas to effuse under identical conditions?
-
Calculate the pressure exerted by 0.5000 mole of N 2 in a 10.000- L container at 25.0 o C. (See Table) a. Use the ideal gas law. b. Use the van der Waals equation. c. Compare the results from parts a...
-
An auditor's audit working papers include the following narrative description of a segment of the Croyden, Inc., factory payroll system and the flowchart presented on the following two pages....
-
What is the logical ending point of a sequential game that starts at position (2,8) with player 1 moving first? Show your work. Player 1 Strategy B Strategy A Strategy A Player 2 Strategy B (3,4)...
-
Problem A-6 Income and Retained Earnings Statements Peanut Corporation is a private corporation using ASPE. At December 31, 2017, an analysis of the accounts and discussions with company officials...
-
8.5 Area Between Curves (dy) Calculus-Calculator Allowed Mastery Check #2 Name: Date: Period: For 1-2, find the area of the region bounded by the following curves. Show the integral set up with...
-
Your company has a travel policy that reimburses employees for the "ordinary and necessary" costs of business travel. Employees often mix a business trip with pleasure by either extending the time at...
-
Simulation A: 1 Diameter 600 mm 2 Focal Length 1800 mm 3 F/D Ratio 3 4 Eyepieces 30 m 5 Barlow? N 6 Celestial Sights M42 - M31 - M51 Simulation B: 1 Diameter 150 mm 2 Focal Length 1800 mm 3 F/D Ratio...
-
The probability that Sam parks in a no-parking zone and gets a parking ticket is 0.06 , and the probability that Sam cannot find a legal parking space and has to park in the noparking zone is 0.20 ....
-
Distinguish among total-moisture content, free-moisture content, equilibrium-moisture content, unbound moisture, and bound moisture.
-
What is the most important aspect of using Automatic Lubers for grease application to a bearing?
-
A certain oxide of titanium is 28.31% oxygen by mass and contains a mixture of Ti2+ and Ti3+ ions. Determine the formula of the compound and the relative numbers of Ti2+ and Ti3+ ions.
-
Spinel is a mineral that contains 37.9% aluminum, 17.1% magnesium, and 45.0% oxygen, by mass, and has a density of 3.57 g/cm3. The edge of the cubic unit cell measures 809 pm. How many of each type...
-
A metallic solid with atoms in a face-centered cubic unit cell with an edge length of 392 pm has a density of 21.45 g/cm3. Calculate the atomic mass and the atomic radius of the metal. Identify the...
-
En prenant un exemple de votre choix, montrer comment on value un swap de taux de change.
-
How much would you need to invest today in order to receive: a. $10,000 in 5 years at 11%? b. $11,000 in 12 years at 8%? c. $12,000 each year for 10 years at 8%? d. $12,000 at the beginning of each...
-
A company that manufactures pulse Doppler insertion flow meters uses the Straight Line method for book depreciation purposes. Newly acquired equipment has a first cost of $190,000 with a 3-year life...

Study smarter with the SolutionInn App


