Carvacrol is a naturally occurring substance isolated from oregano, thyme, and marjoram. What is its IUPAC name?
Question:
Carvacrol is a naturally occurring substance isolated from oregano, thyme, and marjoram. What is its IUPAC name?
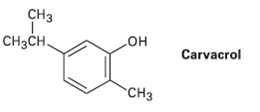
Transcribed Image Text:
CНз но Carvacrol CнзCн. "CHз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
CHCH CH3 ...View the full answer

Answered By

Collins Omondi
I have been an academic and content writer for at least 6 years, working on different academic fields including accounting, political science, technology, law, and nursing in addition to those earlier listed under my education background.
I have a Bachelor’s degree in Commerce (Accounting option), and vast knowledge in various academic fields Finance, Economics, Marketing, Management, Social Science, Women and Gender, Business law, and Statistics among others.
4.80+
4+ Reviews
16+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Trehalose is a naturally occurring disaccharide found in bacteria, insects, and many plants. It protects cells from dry conditions because of its ability to retain water, thereby preventing cellular...
-
Phytane is a naturally occurring alkane produced by the alga Spirogyra and is a constituent of petroleum. The IUPAC name for phytane is 2, 6, 10, 14-tetramethylhexadecane. Write a structural formula...
-
Terreic acid is a naturally occurring antibiotic substance. Its actual structure is an enol isomer of the structure shown. Write the two most stable enol forms of Terreic acid, and choose which of...
-
In May 2020 Bernard, a self-employed plumber, and his son Gerald, a self-employed electrician, purchased 1,000 empty barrels from a Scottish whisky distillery. The barrels were over 100 years old and...
-
What is the resource-based view? Give examples of three different types of resources.
-
The Lakeside Bank is concerned with complaints from customers about its drive-through window operation. Customers complain that it sometimes takes too long to be served. Since there are often cars in...
-
5. Compare a constructive gain on intercompany bonds with an unrealized gain on the intercompany sale of land.
-
Linda Berstler Company sponsors a defined benefit pension plan. The corporations actuary provides the following information about the plan. Instructions (a) Compute the actual return on the plan...
-
K If all other factors are constant, any decrease in fixed costs will decrease the breakeven point. True W False ww
-
Assuming an ideal diode, sketch vi vd,h and id for the half-wave rectifier of Fig. 2.163. The input is a sinusoidal waveform with a frequency of 60 Hz. 0Ideal dc = 2 V 2.2 k
-
Named bombykol, the sex pheromone secreted by the female silkworm moth has the formula C16H280 and the systematic name (10E, 12Z)-10, 12-hexadecadien-1-ol. Draw bombykol showing correct geometry for...
-
What products would you obtain from reaction of 1-penlanol with the following reagents? (a) PBr3 (b) SOCl2 (c) CrO3, H20, H2SO4 (d) PCC
-
According to Figure 8.6, a course can be taught by how many instructors?
-
Based on contract law principles, do you think the jury\'s verdict against the Loewen Group for $ 5 0 0 million was appropriate? Why or why not? What factors should the jury have considered in...
-
5.) Consider you have two systems - one filled with (1kg) water and the other with (1kg) of air. Both systems are at 1000 kPa and 30 C. Determine numerically which fluid system has the larger...
-
Question 3: The partnership of Blossom, Blue, and Kingbird engaged you to adjust its accounting records and convert them uniformly to the accrual basis in anticipation of admitting Kerns as a new...
-
Instructions : Build an Excel spreadsheet using the accounting equation (Assets = Liabilities + Shareholders' Equity). Remember that each transaction has an equal effect on both the left-hand side...
-
7.3 Fill in the spreadsheet below to calculate the port- folio return and risk between Zenon and Dynamics, given the 10 years of annual returns for each stock and portfolio weights of 50/50. (a) How...
-
What do you think? Is there such a thing as ethical hacking?
-
Aztec Furnishings makes hand-crafted furniture for sale in its retail stores. The furniture maker has recently installed a new assembly process, including a new sander and polisher. With this new...
-
The magnitude of one of the following properties must always increase with temperature; that one is (a) Surface tension; (b) Density; (c) Vapor pressure; (d) vap H.
-
Open the 3D Molecular Model on the book's website for buckminsterfullerene. What molecule has its type of ring represented 16 times in the surface of buckminsterfullerene?
-
Draw bond-line formulas and give IUPAC substitutive names for all of the isomers of (a) C4H9Cl and (b) C5H11Br.
-
Give names for the following substituted alkanes: (a) (b) (c) (d) (e) (f) Yo Cl CI OH
-
Jennifer purchased a home for $1,000,000 in 2016. She paid $200,000 cash and borrowed the remaining $800,000. This is Jennifer's only residence. Assume that in year 2024, when the home had...
-
business plan describing company with strengths and weaknesses. Any gaps in plan. Recommendations for improvement of the plan.
-
You wish to buy a car today for $35,000. You plan to put 10% down and finance the rest at 5.20% p.a. for six years. You will make equal monthly payments of $_______.

Study smarter with the SolutionInn App


