Chlorate (CO - 3 ), chlorite (CO - 2 ) , bromate (BrO - 3 ), and
Question:
Chlorate (CO-3), chlorite (CO-2) , bromate (BrO-3), and iodate (IO-3) can be measured in drinking water at the 1-ppb level with 1% precision by selected reaction monitoring. Chlorate and chlorite arise from ClO2 used as a disinfectant. Bromate and iodate can be formed from Br-or I- when water is disinfected with ozone (O3). For the highly selective measurement of chlorate, the negative ion selected by Q1 in Figure 21-26 is m/z 83 and the negative ion selected by Q3 is m/z 67. Explain how this measurement works and how it distinguishes CIO-3 from CIO-2, BrO-3 , and IO-3
Figure 21-6
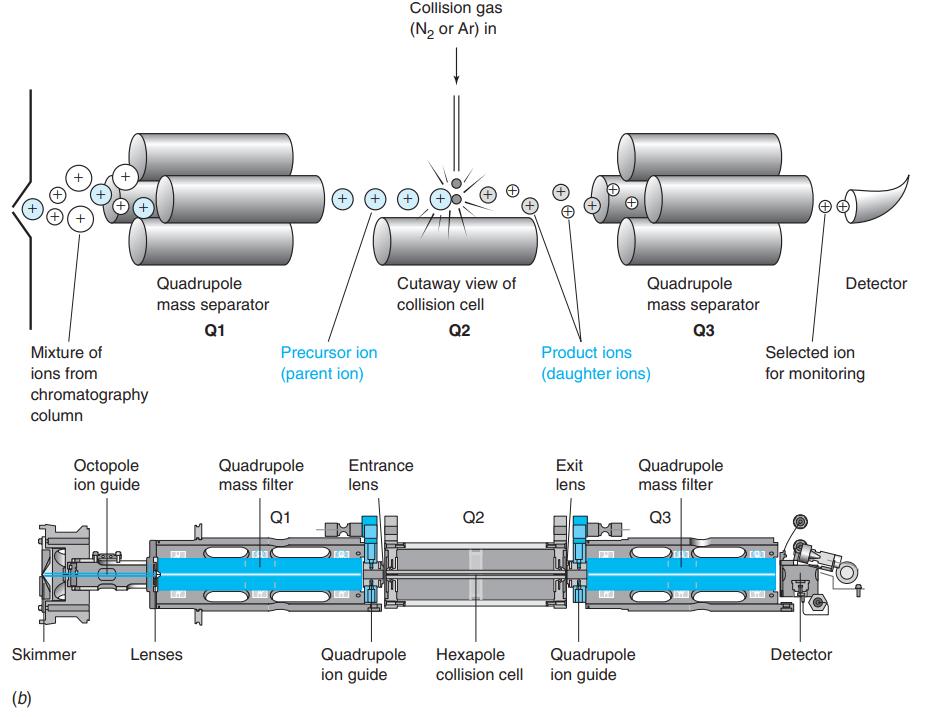
Collision gas (N2 or Ar) in + Quadrupole mass separator Cutaway view of Quadrupole mass separator Detector collision cell Q1 Q2 аз Mixture of Precursor ion Product ions Selected ion ions from (parent ion) (daughter ions) for monitoring chromatography column Octopole ion guide Quadrupole Entrance Exit Quadrupole mass filter mass filter lens lens Q1 Q2 Q3 Skimmer Lenses Quadrupole ion guide Нехарole Quadrupole ion guide Detector collision cell (b)
Step by Step Answer:
Selected reaction monitoring chooses the molecular ion ...View the full answer



Related Video
For this experiment take the bottle halves. Place the bottle upside down in another half of the bottle. Take a piece of cotton and put the cotton in the bottle first. Then pour sand into it. After adding sand pour fine activated charcoal. You can see the making of Homemade ACTIVATED CHARCOAL in the previous video. I’ll give you the link to the video in the description. After making a 1/2 Inch layer of charcoal put 1 inch of gravel layer and then put 2 inches of pebble layer. Repeat the layer-making process in the same manner once again. After this pour dirty water on the top of the bottle. The water we get at the end is free of impurities.
Students also viewed these Chemical Engineering questions
-
The maximum allowable concentration of lead in drinking water is 9.0 ppb.
-
Verify that, in SI units, B/t can be measured in volts-in other words, that 1 Wb/s = 1 V.
-
The true weight of an object can be measured in a vacuum, where buoyant forces are absent. An object of volume V is weighed in air on a balance with the use of weights of density P. If the density of...
-
What sort of debates or experiences would get overlooked if professionals and researchers ignored these distinctions? How would you classify the following? An assigned expatriate who falls in love...
-
You are provided with the following information for Perkins Inc. for the month ended October 31, 2014. Perkins uses a periodic method for inventory. Instructions (a) Calculate (i) ending inventory ,...
-
An upgrade to one of your company's robotics products requires a proportional plus integral compensator that implements the input-output relationship \[ v_{\mathrm{O}}(t)=v_{\mathrm{S}}(t)+50...
-
What are project objectives? AppendixLO1
-
Suppose Consumer Reports would like to conduct a study comparing the prices of televisions made by different manufacturers. The following data show the prices of a random sample of televisions for...
-
The following information pertains to Peak Heights Company: Income Statement for Current Year Sales $ 85,500 Expenses Cost of goods sold $ 51,675 Depreciation expense 8,200 Salaries expense 10,700...
-
The adjusted trial balance columns of the worksheet for Alshwer Company are as follows. Instructions (a) Complete the worksheet by extending the balances to the financial statement columns. (b)...
-
Phytoplankton at the ocean surface maintain the fluidity of their cell membranes by altering their lipid (fat) composition when the temperature changes. When the ocean temperature is high, plankton...
-
In isotope dilution, a known amount of an unusual isotope (called the spike) is added to an unknown as an internal standard for quantitative analysis. After the mixture has been homogenized, some of...
-
The price-earnings ratio of McDonnell Douglas (air-craft builder) was 5 , and the price-earnings ratio ofMicrosoft (computer software) was 43. Which com-pany did the stock market favor? Explain.
-
In the exchange lemma for the scheduling problem, we say that the first event to finish a* in a given time period [i,j] is always part of the optimal solution for that same time period. To argue...
-
4 10 points Company's year-end is December 31. Calculate depreciation for each year of the machine's estimated useful life under each of the following methods: (Do not round intermediate...
-
3. The walls of an oven are made from steel sheets with insulating board between them of thermal conductivity 0.18 J m-1 s -1 C-1 . If the maximum internal temperature in the oven is 300C and the...
-
Egyptian Spa produces two different spa products: Relax and Refresh. The company uses three operations to manufacture the products: mixing, blending, and packaging. Because of the materials used,...
-
Part A At a given instant A has the motion shown in (Figure 1). Determine the acceleration of B at this instant. Express your answer in feet per second squared to three significant figures. Enter...
-
Histidine is an essential amino acid that the body uses to form proteins. The Lewis structure of histidine follows. What are the approximate values for bond angles 1 through 5 (indicated on the...
-
A superior criticized a sales manager for selling high-revenue, low-profit items instead of lower-revenue but higher-profit items. The sales manager responded, My income is based on commissions that...
-
(a) What are the advantages and disadvantages of using a narrower open tubular column? (b) What are the advantages and disadvantages of using a longer open tubular column? (c) What are the advantages...
-
(a) What types of solutes are typically separated with a poly(dimethylsiloxane)-coated open tubular column? (b) What types of solutes are typically separated with a poly(ethylene glycol)-coated open...
-
(a) What are the advantages and disadvantages of temperature programming in gas chromatography? (b) What is the advantage of pressure programming?
-
Your company BMG Inc. has to liquidate some equipment that is being replaced. The originally cost of the equipment is $120,000. The firm has deprecated 65% of the original cost. The salvage value of...
-
1. What are the steps that the company has to do in time of merger transaction? And What are the obstacle that may lead to merger failure? 2.What are the Exceptions to not to consolidate the...
-
Problem 12-22 Net Present Value Analysis [LO12-2] The Sweetwater Candy Company would like to buy a new machine that would automatically "dip" chocolates. The dipping operation currently is done...

Study smarter with the SolutionInn App


