Choline, a component of the phospholipids in cell membranes, can be prepared by SN2 reaction of trimethyl
Question:
Choline, a component of the phospholipids in cell membranes, can be prepared by SN2 reaction of trimethyl amine with ethylene oxide. Show the structure of choline, and propose a mechanism for thereaction.
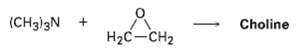
Transcribed Image Text:
(CHд])3N + Choline H2C-CH2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 68% (22 reviews)
The reaction of trimethylamin...View the full answer

Answered By

Shadrack Mulunga
I am a Biochemistry by profession. However, I have explored different fields of study. My quest to explore new fields has helped me gain new knowledge and skills in Business, clinical psychology, sociology, organizational behavior and general management, and Project Management. I count my expertise in Project management, in particular, creation of Work Break Down Structure (WBS) and use of Microsoft Project software as one of my greatest achievement in Freelancing industry. I have helped thousands of BSC and MSC students to complete their projects on time and cost-effectively using the MS Project tool. Generally, I find happiness in translating my knowledge and expertise to success of my clients. So far, i have helped thousands of students to not only complete their projects in time but also receive high grades in their respective courses. Quality and timely delivery are the two key aspects that define my work. All those who hired my services always come back for my service. If you hire my services today, you will surely return for more. Try me today!
5.00+
154+ Reviews
289+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Ethers can often be prepared by S N 2 reaction of alkoxide ions, RO ? , with alkyl halides. Suppose you wanted to prepare cyclohexyl methyl ether. Which of the two possible routes shown below would...
-
A compound known as Hagemann's ester can be prepared by treating a mixture of formaldehyde and ethyl acetoacetate first with base and then with acid and heat. Write the structure for the product of...
-
Choline (Sec. 11.11) can be prepared by the reaction of trimethylamine with ethylene oxide. Write an equation for the reaction, and show its mechanism.
-
1. An auditor selected a product maintained in the finished goods Warehouse. The auditor counted the product and compare this amount what the amount in the finished goods Perpetual inventory...
-
Finding job openings that align perfectly with your professional interests is wonderful, but it doesn't always happen. Sometimes you have to widen your search and go after whatever opportunities...
-
Unpolarized light of intensity I 0 is incident on three polarizing filters. The axis of the first is vertical, that of the second is 45 from vertical, and that of the third is horizontal. What light...
-
What are the most serious consequences of the new contractual clause that ties input (labor hours) to output (number of meals/meal equivalents served)?
-
Benson Oil is being considered for acquisition by Dodd Oil. The combination, Dodd believes, would increase its cash inflows by $25,000 for each of the next 5 years and by $50,000 for each of the...
-
e Direct 26 XYZ Company's accountant is estimating next period's total overhead costs (Y). She performed three regression analyses, the first is based on direct labor hours (DLH), the second is based...
-
The following salaried employees of Mountain Stone Brewery in Fort Collins, Colorado, are paid semimonthly. Some employees have union dues or garnishments deducted from their pay. Calculate their net...
-
Chlorophyll, heme, vitamin B12, and a host of other substances are bio-synthesized from porphohilinogen (PBG), which is itself formed from condensation of two molecules of 5-aminolevulinate. The two...
-
Cyclopentamine is an amphetamine-like central nervous system stimulant. Propose a synthesis of Cyclopentamine from materials of five carbons orless. CH3 -CH2CHNHCH3 Cyclopentamine
-
Show that tan 1 (1/v)= /2 tan 1 v, if v > 0.
-
The following data apply to Superior Auto Supply Inc. for May 2011. 1. Balance per the bank on May \(31, \$ 8,000\). 2. Deposits in transit not recorded by the bank, \(\$ 975\). 3. Bank error; check...
-
How do you determine whether there is a linear correlation between two variables \(x\) and \(y\) ? Use Table 14.10. Table 14. 10 n a = 0.05 0.950 0.878 4 5 6 0.811 7 0.754 8 0.707 9 0.666 10 0.632 11...
-
Comparative Analysis Problem: Columbia Sportswear Company vs. Under Armour, Inc. The financial statements for the Columbia Sportswear Company can be found in Appendix A and Under Armour, Inc.'s...
-
The following information is available for Book Barn Company's sales on account and accounts receivable: After several collection attempts, Book Barn wrote off \(\$ 4,500\) of accounts that could not...
-
The following information comes from the accounts of Jersey Company: Required a. There were \(\$ 170,000\) of sales on account during the accounting period. Write-offs of uncollectible accounts were...
-
Identify key hospitality industry unions and their areas of geographic relevance.
-
Find a polar equation for the curve represented by the given Cartesian equation. 4y 2 = x
-
What is a general characteristic of a Lewis acid? Of a Lewis base?
-
Will 2-hexyne react with sodium amide? Explain.
-
For the following compounds, write structural formulas and IUPAC names for all possible isomers having the indicated number of multiple bonds: a. C4H6 (one triple bond) b. C5H10 (one double bond) c....
-
Name the following compounds by the IUPAC system: a. CH3CH=C(CH2CH2CH3)2 b. (CH3)2CHCH"CHCH3 c. g. CH3-C-C-CH-CH, h. k.
-
During 2024, its first year of operations, Hollis Industries recorded sales of $11,900,000 and experienced returns of $760,000. Cost of goods sold totaled $7,140,000 (60% of sales). The company...
-
What is the value of a 15% coupon bond with 11% return? Is it a discount or a premium bond?
-
A manufacturer with a December 31 taxation year end sells new machinery for $50,000 on January 2, 2022. The cost of the machinery is $20,000. The terms of the sale require an initial payment of...

Study smarter with the SolutionInn App


