Ethers can often be prepared by S N 2 reaction of alkoxide ions, RO ? , with
Question:
Ethers can often be prepared by SN2 reaction of alkoxide ions, RO?, with alkyl halides. Suppose you wanted to prepare cyclohexyl methyl ether. Which of the two possible routes shown below would you choose? Explain.
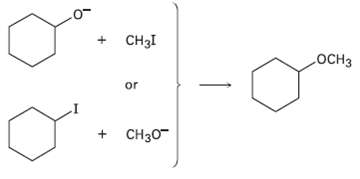
Transcribed Image Text:
CH3I .ОCHЗ or + CH30
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
CH3I OCH3 CH30 This is an excellent method of ether preparat...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Suppose you wanted to test H0: p = 0.50, but you had 0 successes in n trials. If you had found the test statistic using the se = p(1 - p)/n designed for confidence intervals, show what happens to the...
-
Suppose you wanted to know if men or women students spend more money on clothes. You consider two different plans for carrying out an observational study: Plan 1: Ask the participants how much they...
-
Suppose you wanted to test your extrasensory perception (ESP) ability using an ordinary deck of 52 cards, which has 26 red and 26 black cards. You have a friend shuffle the deck and draw cards at...
-
Identify how balance sheet quality and earnings quality were impaired in each of the following accounting scandals: Waste Management 1988 - Falsely increased the useful lives of long-lived tangible...
-
Keeping in mind that an agency like LTSC would have limited funding and just a few senior managers, suggest two additional developmental activities that are likely to be most beneficial to the...
-
Diversified Industries plc (DI) is a business that has interests in engineering, caravan manufacturing and a chain of shops selling car accessories. DI has recently been approached by the directors...
-
Using subjective performance standards is best when designing a rating scale performance appraisal form. A. True B. False
-
Jane Marks has a restaurant in which she accepts credit cards and checks. Several of the places that Jane shops now accept debit cards and do not accept checks. Jane's banker explained that a debit...
-
ReQuired answer for Risk 2 and risk 3 This week in Chapter 5 we identified different types of audit procedures that the auditor may perform when conducting the audit engagement, as well as different...
-
A fabric mill has developed the following forecasts (in hundred bolts of cloth). The mill has a normal capacity of 275 units (a unit equals one hundred bolts) per month, and employs 275 workers....
-
Reaction of the following S tosylate with cyanide ion yields a nitrile product that also has S stereochemistry.Explain. TOS NaCN |" CH2OCH3 (S stereochemistry)
-
We saw in Section 7.8 that bromohydrin are converted into epoxides when treated with base. Propose a mechanism, using curved arrows to show the electronflow. Br -C CH3 CH NAOH Ethanol TH CH C
-
A circular test track for cars in England has a circumference of 3.2 km. A car travels around the track from the southernmost point to the northernmost point. a. What distance does the car travel? b....
-
What is the amount of Gain or Loss recognized on the disposition? Enter a Gain as a positive number or a Loss as a negative number or Zero if neither is recognized. R&R purchased a piece of equipment...
-
B)There is a significant increase in Machinery, Equipment, and Office Furniture (576%), a significant increase in A significant increase in the Line of Credit (344%), and a significant increase in...
-
Ryvel Company has 2 (two) Production Departments, namely Department I and Department II. In addition, it has 2 (two) Supporting Departments, namely Department C and Department D. Ryvel Company...
-
Dollars According to the graph below, what should this profit-maximizing firm do? P3 P4 MC ATC 0 Q3Q1Q2 MR D Quantity
-
Nyameye Ent. Manufactures rubber at Kurriasi. The following details relate to the movement of materials in February 2010. February Beginning balance: 800 units @ $6 per unit. 5 Received 200 units $7...
-
explain how conventions may conflict with each other;
-
Annual dividends of ATTA Corp grew from $0.96 in 2005 to $1.76 in 2017. What was the annual growth rate?
-
Consider the reaction: An equilibrium mixture of this reaction at a certain temperature has [NH 3 ] = 0.278 M and [H 2 S] = 0.355 M. What is the value of the equilibrium constant (K c ) at this...
-
(a) Propose a mechanism for the reaction of benzyl alcohol with acetyl chloride to give benzyl acetate. (b) Propose a mechanism for the reaction of benzoic acid with acetyl chloride to give acetic...
-
The mass spectra of acid derivatives follow the principles shown in Chapter 18 for other carbonyl compounds and for alkoxy groups. Both McLafferty rearrangements and alpha-cleavages are common. The...
-
An unknown compound gives a mass spectrum with a weak molecular ion at m/z 113 and a prominent ion at m z 68. Its NMR and IR spectra are shown here. Determine the structure, and show how it is...
-
Assume that an investment of $100,000 is expected to grow during the next year by 8% with SD 20%, and that the return is normally distributed. Whats the 5% VaR for the investment? A. $24,898 B....
-
Simpson Ltd is a small IT company, which has 2 million shares outstanding and a share price of $20 per share. The management of Simpson plans to increase debt and suggests it will generate $3 million...
-
The following are the information of Chun Equipment Company for Year 2 . ( Hint: Some of the items will not appear on either statement, and ending retained earnings must be calculated. ) Salaries...

Study smarter with the SolutionInn App


