We saw in Section 7.8 that bromohydrin are converted into epoxides when treated with base. Propose a
Question:
We saw in Section 7.8 that bromohydrin are converted into epoxides when treated with base. Propose a mechanism, using curved arrows to show the electronflow.
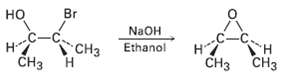
Transcribed Image Text:
Br но -C CH3 CHз н NAOH Ethanol TH CHз CНз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 52% (17 reviews)
HO Br 1 rotate HCH3 180 H HC H HO CH3 CC H H3C Br ...View the full answer

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Organic Chemistry questions
-
Bromohydrins are converted into epoxides when treated with base. Draw curved arrows to indicate the flow of electrons in this step of the mechanism for the below reaction. : : Br :: Br "CH . " H3C
-
Draw curved arrows to show the flow of electrons responsible for the conversion of reactants into products. a. b. c. :O CH-C OCH
-
We saw in Section 11.5 that, to find the energies of the bonding and antibonding orbitals of a heteronuclear diatomic molecule, we need to solve the secular determinant where aA et aB and we have...
-
Mr. Jalal Talabani is a resident of Bangladesh. The information regarding his investments in securities during the income year 2019-20 are as follows: a) 15% Beximco Debentures were purchased from...
-
Imagine that LTSC called you in as a consultant before Watanabe started his sabbatical. The agency has asked you to help obtain the maximum developmental benefit from the sabbatical arrangement. How...
-
The financial statements of Freezeqwik Ltd, a distributor of frozen foods, are set out below for the year ended 31 December last year. All purchases and sales are on credit. There has been no change...
-
Formal employee performance appraisals are normally conducted once or twice per year. A. True B. False
-
Working backward to consolidation relations. Laesch Company, as parent, owns shares in Lily Company. Laesch has owned the shares since it formed Lily. Lily has never declared a dividend. Laesch has...
-
With steps please.. On June 30, 2018, Roddick Company had a cash balance in its general ledger of $11,595. The company's bank statement from Bank One showed a June 30 balance of $12,540. The...
-
Allocation for Economic Decisions and Motivation: Bonn Company recently reorganized its computer and data processing activities. The small installations located within the accounting departments at...
-
Ethers can often be prepared by S N 2 reaction of alkoxide ions, RO ? , with alkyl halides. Suppose you wanted to prepare cyclohexyl methyl ether. Which of the two possible routes shown below would...
-
Show the stereochemistry of the epoxide you would obtain by formation of a bromohydrin from trans-2-butene, followed by treatment with base.
-
In 1972, a worker at a nuclear fuel plant in France found that uranium from a mine in Oklo, in the African Republic of Gabon, had less U-235 than the normal 0.7% a quantity known from meteorites and...
-
On January 1, 20X1, Popular Creek Corporation organized SunTime Company as a subsidiary in Switzerland with an initial investment cost of Swiss francs (SFr) 76,000. SunTime's December 31, 20X1, trial...
-
In November 2 0 2 4 , Lily informed you that she needs additional cash flow to meet her personal debt obligations. Lily does not want to sell more stock than she needs to because she wants...
-
Bennett limited provides mobile library services to the community of longbourn. bennett has preliminary operating results for the first year and the company found that net income is different from...
-
What is printed when the value of x is 34? if (x < 32 ) { if (x22) { } System.out.println("Blue"); else if (x <10) { } System.out.println("Red"); else if (x > 25 ) { System.out.println("Yellow"); } }...
-
Heidi expresses concern in the video that when employees are also friends, holding them accountable for performance "doesn't come as naturally" to her. She asks you, "Does my focus on relationship...
-
explain what is meant by a 'firm';
-
Assume that your audit team has established the following parameters for the examination of ELM's sales transactions: LO G-3 Risk of incorrect acceptance...
-
Consider the reaction: An equilibrium mixture of this reaction at a certain temperature has [CO] = 0.105 M, [H 2 ] = 0.114 M, and [CH 3 OH] = 0.185 M. What is the value of the equilibrium constant (K...
-
An unknown compound gives the NMR, IR, and mass spectra shown next. Propose a structure, and show how it is consistent with the observed absorptions. Show fragmentations that account for the...
-
The IR spectrum, 13C NMR spectrum, and 1HNMR spectrum of an unknown compound (C6H8O3) appear next. Determine the structure, and show how it is consistent with the spectra. wavelength (um) 5 5.5 6 710...
-
An unknown compound of molecular formula C5H9NO gives the IR and NMR spectra shown here. The broad NMR peak at δ7.55 disappears when the sample is shaken with D2O. Propose a structure,...
-
How do warehouses and distribution centers differ? What is cross-docking and why might a company choose to cross-dock a product? What kinds of products can be delivered electronically? What kinds...
-
Strawberry Inc. has historically been an all-equity firm. The analyst expects EBIT to be $1.5B in perpetuity starting one year from now. The cost of equity for the company is 11.5% and the tax rate...
-
Guzman company received a 60- day, 5 % note for 54,000 dated July 12 from a customer on account. Determine the due date on note. Determine the maturity value of the note and journalize the entry of...

Study smarter with the SolutionInn App


