Cinnamaldehyde, the aromatic constitution of cinnamon oil, can be synthesized by a mixed aldol condensation. Show the
Question:
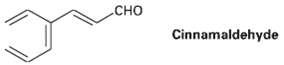
Cinnamaldehyde, the aromatic constitution of cinnamon oil, can be synthesized by a mixed aldol condensation. Show the starting materials you would use, and write thereaction.
Transcribed Image Text:
сно CHO Cinnamaldehyde
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (18 reviews)
CHO CH3CHO 1 NaOH EtOH 2 heat ...View the full answer

Answered By

MICHAEL KICHE
I was employed studypool for the first time in tutoring. I did well since most of my students and clients got the necessary information and knowledge requested for. I always submitted the answers in time and followed the correct formatting in answering eg MLA or APA format,
Again I worked with the writers bay where I did writing and got many clients whom we worked with so closely. They enjoyed every single service I delivered to them. My answers are always correct.
4.70+
13+ Reviews
54+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The following compounds can be synthesized by aldol condensations, followed by further reactions. (In each case, work backward from the target molecule to an aldol product, and show what compounds...
-
Epoxides can be synthesized by treating halohydrins with aqueous base. Propose a mecha-nism for reactions (a) and (b), and explain why no epoxide formation is observed in (c). (a) (b) (c) CI NaOH H20...
-
Vicinal halo alcohols (halohydrins) can be synthesized by treating epoxides with HX. (a) Show how you would use this method to synthesize 2-chlorocyclopentanol from cyclopentene. (b) Would you expect...
-
4. Jerry intends to use the money from his loan (and his personal savings if necessary) to make an investment in his friend Elaines business. In return, Elaine has predicted the following returns on...
-
Suppose you were granted a "risky job" of the type studied in this chapter. The job pays $40,000 with probability 1/2, and $160,000 with probability 1/2. What is your certainty equivalent for this...
-
Figure EX26.9 shows a graph of V versus x in a region of space. The potential is independent of y and z. What is E x at (a) X = -2 cm, (b) X = 0 cm, (c) X = 2 cm? V (V) 10- x (m) -32 -1 1 2 3 107...
-
What is the difference between job descriptions and job specifications? LO.1
-
Why cant we pay our shareholders a dividend? Shouts your new boss at Polar Opposites, This income statement you prepared for me says we earned $5million in our first year! You recently prepared the...
-
Name the sections of the Statement of Cash Flows? A. Income Statement Activities, Statement of Account Activities, Balance Sheet Activities, General Ledger Activities B. Investing Activities,...
-
Pacifico Company, a U.S.-based importer of beer and wine, purchased 1,000 cases of Oktoberfeststyle beer from a German supplier for 50,000 euros. Relevant U.S. dollar exchange rates for the euro are...
-
How can you account for the fact that 2, 2, 6-trimethylcyclohexanone yields no detectable aldol product even though it has an acidic hydrogen?
-
The bicycle ketone shown below does not undergo aldol self-condensation even though it has two ? hydrogen atoms. Explain.
-
What is meant by a size threshold?
-
What is the formula for Bouley's coefficient of skewness?
-
What is the relation between orthocentre,circumcentre and centroid of a triangle?
-
When do we use Fourier transforms and Laplace transforms in RC/RL/RLC circuit analysis?
-
What are the protocols used in a drone?
-
How do we design a drone?
-
When management receives Rons report, what do you think the reaction will be? Why? Do you feel that management should discipline Luke? If so, how exactly should they approach him? Should the contents...
-
After looking at the resources, explain what a spirit image is. Why might looking at a god and/or a human in terms of their spirit be helpful if you want to eliminate some of the divisions between...
-
In N 2 O, nitrogen is the central atom, and the oxygen atom is terminal. In OF 2 , however, oxygen is the central atom. Use formal charges to explain why. Formal charge = number of valence electrons...
-
Give the expected product(s) of E2 elimination for each reaction. (Hint: Use models!) (a) (b) CH, H NaOCH one product HC H NaOCH two products
-
Determine the number of elements of unsaturation in the molecular formula C4H6. Give all nine possible structures having this formula. Remember that A double bond = one element of unsaturation A ring...
-
The preceding example shows meso-1,2-dibromo-1,2-diphenylethane reacting with iodide ion to give trans-stilbene. Show how the other diastereomer of the starting material gives a different...
-
1. Why might managers of small restaurants decide not to adopt the standard work hour approach to controlling labour cost? (minimum 150 words )
-
Which statement is true regarding the U.S. GAAP impairment test for limited life intangibles? A. U.S. GAAP impairment is likely to be greater than IFRS impairment. B. The impairment test for limited...
-
Which of the following is a limitation of both return on investment and residual income? A. Favors large units. B. There is disincentive for high return on investment units to invest. C. Can lead to...

Study smarter with the SolutionInn App


