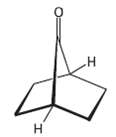
The bicycle ketone shown below does not undergo aldol self-condensation even though it has two ? hydrogen
Question:
The bicycle ketone shown below does not undergo aldol self-condensation even though it has two ? hydrogen atoms. Explain.
Transcribed Image Text:
エ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (14 reviews)
The first step of an aldol condensation is enolate for...View the full answer

Answered By

Asim farooq
I have done MS finance and expertise in the field of Accounting, finance, cost accounting, security analysis and portfolio management and management, MS office is at my fingertips, I want my client to take advantage of my practical knowledge. I have been mentoring my client on a freelancer website from last two years, Currently I am working in Telecom company as a financial analyst and before that working as an accountant with Pepsi for one year. I also join a nonprofit organization as a finance assistant to my job duties are making payment to client after tax calculation, I have started my professional career from teaching I was teaching to a master's level student for two years in the evening.
My Expert Service
Financial accounting, Financial management, Cost accounting, Human resource management, Business communication and report writing. Financial accounting : • Journal entries • Financial statements including balance sheet, Profit & Loss account, Cash flow statement • Adjustment entries • Ratio analysis • Accounting concepts • Single entry accounting • Double entry accounting • Bills of exchange • Bank reconciliation statements Cost accounting : • Budgeting • Job order costing • Process costing • Cost of goods sold Financial management : • Capital budgeting • Net Present Value (NPV) • Internal Rate of Return (IRR) • Payback period • Discounted cash flows • Financial analysis • Capital assets pricing model • Simple interest, Compound interest & annuities
4.40+
65+ Reviews
86+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The following compound is not aromatic even though it has 4n + 2 electrons in a continuous cyclic array. Explain why this compound is not aromatic.
-
The trial balance of Dominic Company shown below does not balance. Your review of the ledger reveals that each account has a normal balance. You also discover the following errors. 1. The totals of...
-
The trial balance of Zoop Co. shown below does not balance. Each of the listed accounts has a normal balance per the general ledger. An examination of the ledger and journal reveals the following...
-
1. What responsibility does an organization have to ensure that its suppliers and business partners behave ethically? To whom is this responsibility owed? 2. How can an organization monitor the...
-
In the United States, lawyers in negligence cases are usually paid a contingency fee equal to roughly 30 percent of the total award. Lawyers in other types of cases are often paid on an hourly basis....
-
Figure Q26.8 shows a negatively charged electroscope. The gold leaf stands away from the rigid metal post. Is the electric potential of the leaf higher than, lower than, or equal to the potential of...
-
Define the following: a. Jobanalysis b. Job description c. Job specifications d. Jobdesign LO.1
-
As the media landscape continues to change, and advertisers shift more and more of their monies into new media, there are those who predict that some traditional media like TV and newspapers may not...
-
Knowledge Check 01 Which of the following factors should be considered when deciding whether to keep a product line or drop it? (Select all that apply) Check All That Apply 0 Opportunity costs of...
-
1. The textbook lists 11 major community factors to use as selection criteria for choosing a location. Mary realizes that all factors are important; however, 11 are too many because it waters down...
-
Cinnamaldehyde, the aromatic constitution of cinnamon oil, can be synthesized by a mixed aldol condensation. Show the starting materials you would use, and write thereaction. CHO Cinnamaldehyde
-
What condensation product would you expect to obtain by treatment of the following substances with sodium ethoxide in ethanol? (a) Ethyl Butanoate (b) Cyclopentanone (c) 3, 7-Nonanedione (d)...
-
A sentinel event is an unexpected occurrence involving death or serious physical or psychological injury, or the risk thereof. The Joint Commission encourages self-reporting of sentinel events.
-
What are the different types of drones?
-
What are the applications of drones?
-
What are the various protocols in telecom domain?
-
What are the various types of routing protocols?
-
For all the benefits they bring to business, social media and other communication technologies have created a major new challenge: responding to online rumors and attacks on a company's reputation....
-
If management decides to terminate Luke based upon the contents of the mystery shoppers report, what assurances are needed from the mystery shopper company? Why?
-
What are the main distinctions between the different schools of legal interpretation?
-
Use formal charge to identify the better Lewis structure. H H-C= H H=C
-
Solved Problem 7-4 showed that the debromination of (R,R)-2,3-dibromobutane gives cis-but- 2-ene. Draw the same reaction using the (S,S) enantiomer and show that it gives the same diastereomer of the...
-
Predict the elimination products formed by debromination of the following compounds with iodide ion in acetone. Include stereochemistry, and give a correct name for each product. (a)...
-
The following compounds show different rates of debromination. One reacts quite fast, and the other seems not to react at all. Explain this surprising difference in rates. Br KI, acetone Br (CH),C RS...
-
exercise 4-7 (Algo) Effects of transactions on income statement LO P2
-
Compute the value of ordinary bonds under the following circumstances assuming that the coupon rate is 0.06:(either the correct formula(s) or the correct key strokes must be shown here to receive...
-
A tax-exempt municipal bond has a yield to maturity of 3.92%. An investor, who has a marginal tax rate of 40.00%, would prefer and an otherwise identical taxable corporate bond if it had a yield to...

Study smarter with the SolutionInn App


