Use formal charge to identify the better Lewis structure. H H-C= H H=C
Question:
Use formal charge to identify the better Lewis structure.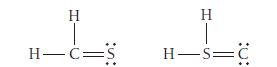
Transcribed Image Text:
H H-C= H H=C
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
H HCS 00 ...View the full answer

Answered By

OTIENO OBADO
I have a vast experience in teaching, mentoring and tutoring. I handle student concerns diligently and my academic background is undeniably aesthetic
4.30+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A long conducting wire made of anti-matter carries a uniform current per unit area. The anti-electrons (each carries charge +1.602 x 10-19 C ) with speed 4.862 m/s (the speed and magnitude of the...
-
Use formal charges to identify the better Lewis structure. H H-S-C-H 1 H H T H-C-S-H I H
-
LMO Company incurred expenditures for leasehold improvements during the year. The relevant lease is on an office building that the Company rented at the beginning of 2021. The improvements cost...
-
Consider the many moments of joy in the movie. Why include them? What risky acts of courage do Katherine, Mary and Dorothy take? How are they rewarded? The film shows the strong relationships that...
-
What is online analytical processing? How is OLAP related to databases? What is a pivot table, and how are pivot tables and OLAP related?
-
Indicate whether each of the following statements is true or false by writing T or F in the answer c olumn. The sole purpose of administrative agencies is to regulate business fi rms.
-
7 Up using variances to read the market. The following is an excerpt from an article that appeared in a recent issue of a trade magazine: Remember about 30 years back, when 7 Up began describing...
-
Eve Dalton is the new controller for Smashing Hits, a designer and manufacturer of tennis attire. Shortly before the December 31 fiscal year- end, Liz Sinclair (the company president) asks Dalton how...
-
10. A Company issued common stock to investors and received $10,000. Choose the TRUE statement A. For this transaction, cash increases and common stock decreases. B. The journal entry to record this...
-
You are evaluating the HomeNet project under the following assumptions: new tax laws allow 100% bonus depreciation (all the depreciation expense, $7.5 million, occurs when the asset is put into use,...
-
In N 2 O, nitrogen is the central atom, and the oxygen atom is terminal. In OF 2 , however, oxygen is the central atom. Use formal charges to explain why. Formal charge = number of valence electrons...
-
Write a Lewis structure that obeys the octet rule for each ion. Include resonance structures if necessary and assign formal charges to each atom. a. CIO3 b. ClO4 c. NO3 + d. NH4
-
Given that you wanted to design a second-order Markov model, i.e., where each observable state would be dependent on the previous two observable states. How would you do this? What would the...
-
Main Street Cinema invited three firms to bid on its daily janitorial services contract, and then scored those tenders based on quality, reliability/risk, and price (using a 100-point scale in each...
-
Pick two of the industries listed in Exhibit 1, one on the high end of profitability and one on the low end. What are the boundaries of these industries? What are their market and geographic...
-
Assume the following information (figures are in thousands): a. Calculate the monthly budget and monthly cumulative budgets for the project. b. Draw a project S-curve representation of this project....
-
Assume that you are a project manager in a software firm and have been asked to calculate the expected cost for a new fast-food epos system. Referring to historical information, you know that the...
-
Dormant eggs of the zooplankton Daphnia survive in lake sediments for decades, making it possible to measure their physiological traits in past years. Hairston et al. (1999) extracted Daphnia eggs...
-
For the aluminum alloy, whose stress strain behavior may be observed in the "Tensile Tests" module of Virtual Materials Science and Engineering (VMSE), determine the following: (a) the approximate...
-
Suppose you need to answer any four of seven essay questions on a history test and you can answer them in any order. a. How many different question combinations are possible? b. What is the...
-
What is the barometric pressure reading in millimeters of mercury corresponding to 101.325 kPa(abs)?
-
Why must a barometric pressure reading be corrected for temperature?
-
By how much would the barometric pressure reading decrease from its sea-level value at an elevation of 1250 ft?
-
If you made a fixed deposit of $10,000 with an annual interest rate of 3% but the rate of inflation for that year is 3% as well, the calculation of Real Interest Rate would be like this
-
Miller Brothers Hardware paid an annual dividend of $1.80 per share last month. Today, the company announced that future dividends will be increasing by 3.20 percent annually. If you require a 9.5...
-
We know that possessing common stocks represents the corresponding ownership of that share of the companys assets. Suppose an investor buys 1% of equity of a levered firm, then her payoff will be A....

Study smarter with the SolutionInn App


