Use formal charges to identify the better Lewis structure. H H-S-C-H 1 H H T H-C-S-H I
Question:
Use formal charges to identify the better Lewis structure.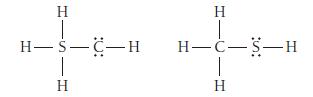
Transcribed Image Text:
H H-S-C-H 1 H H T H-C-S-H I H
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
To determine the better Lewis structure based on formal charges we look for the structure in which t...View the full answer

Answered By

Grace Igiamoh-Livingwater
I am a qualified statistics lecturer and researcher with an excellent interpersonal writing and communication skills. I have seven years tutoring and lecturing experience in statistics. I am an expert in the use of computer software tools and statistical packages like Microsoft Office Word, Advanced Excel, SQL, Power Point, SPSS, STATA and Epi-Info.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
A long conducting wire made of anti-matter carries a uniform current per unit area. The anti-electrons (each carries charge +1.602 x 10-19 C ) with speed 4.862 m/s (the speed and magnitude of the...
-
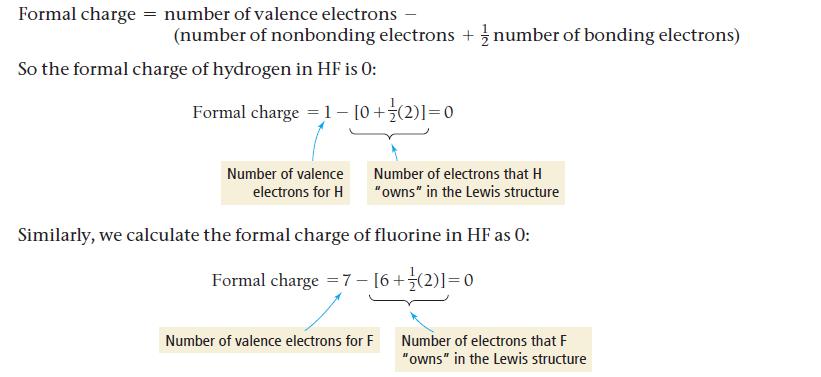
In N 2 O, nitrogen is the central atom, and the oxygen atom is terminal. In OF 2 , however, oxygen is the central atom. Use formal charges to explain why. Formal charge = number of valence electrons...
-
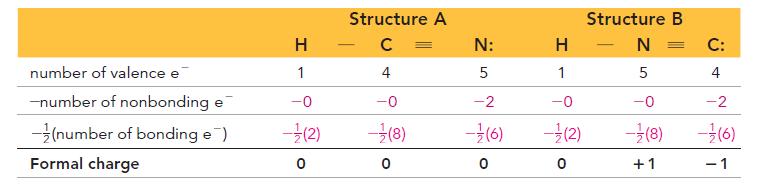
Use formal charge to identify the better Lewis structure. H H-C= H H=C
-
Provide the appropriate statute for your answer (format style - IRAC - Issue, Rule, Application and Conclusion.) what are your thought? Problem Scenario- Earnest is married to Janice. Earnest and...
-
What is SQL? How is SQL like an Access query? How is it different?
-
Indicate whether each of the following statements is true or false by writing T or F in t he a nswer c olumn. Vicarious liability means that one person can be held responsible for the negligent acts...
-
Variance analysis of revenues, multiple products. The Penguins play in the North American Ice Hockey League. The Penguins play in the Downtown Arena, which has a capacity of 30,000 seats (10,000...
-
Newton Company introduced a line of laptop computers in 2016 that carry a 1-year assurance-type warranty. Based on experience with other computers that it sells, Newton estimated warranty costs as 3%...
-
Trio Company reports the following information for the current year, which is its first year of operations. Assume instead that Trio Company uses variable costing. (Round intermediate calculations...
-
Barbour Corporation, located in Buffalo, New York, is a retailer of high-tech products and is known for its excellent quality and innovation. Recently, the firm conducted a relevant cost analysis of...
-
How important is the resonance structure shown here to the overall structure of carbon dioxide? Explain. :0=C:
-
Write a Lewis structure that obeys the octet rule for each ion. Include resonance structures if necessary and assign formal charges to each atom. a. CIO3 b. ClO4 c. NO3 + d. NH4
-
Explain how a non-controlling shareholder in a subsidiary would use the non-controlling interest accounts on the parent's consolidated balance sheet and income statement.
-
For each of the following events, determine the amount of freight paid by The Book Shop. Also indicate whether the freight cost would be classified as a product or period (selling and administrative)...
-
The following account titles and balances were taken from the adjusted trial balance of Scoggins Sales Co. at December 31, 2011. The company uses the periodic inventory method. Required a. Prepare a...
-
Teds Taxi Company (TTC) is considering the purchase of four new taxicabs. Various information about the proposed investment follows: Required: Help TTC evaluate this project by calculating each of...
-
Dayton Corp has \($2\) million to invest in new projects. The companys managers have presented a number of possible options that the board must prioritize. Information about the projects follows:...
-
Glowbright Company makes three types of long-burning scented candles. The models vary in terms of size and type of materials (fragrance, decorations, etc.). Unit information for Glowbright follows:...
-
For the tempered steel alloy, whose stress strain behavior may be observed in the "Tensile Tests" module of Virtual Materials Science and Engineering (VMSE), determine the following: (a) the...
-
For liquid water the isothermal compressibility is given by; where r and b are functions of temperature only. If 1 kg of water is compressed isothermally and reversibly from I to 500 bar at 60(C. how...
-
Denver, Colorado, is called the Mile-High City because it is located at an elevation of approximately 5200 ft. Assuming that the sea-level pressure is 101.3 kPa(abs), what would be the approximate...
-
The barometric pressure is reported to be 28.6 in of mercury. Calculate the atmospheric pressure in psia.
-
A barometer indicates the atmospheric pressure to be 30.65 in of mercury. Calculate the atmospheric pressure in psia.
-
GM has a current stock price of $97.88. If they issued a dividend of $3.18 last week, and the dividend is projected to grow at 4.9% what is the cost of equity capital for GM? Please provide all...
-
Show your work Has anything similar to Earth's weather patterns been seen on other planets
-
I am struggling to answer the rest of REQUIREMENTS 5-9. Please assist if possible. I have included an example of a worked-out problem for a reference and then under I will post my actual problem that...

Study smarter with the SolutionInn App


