Consider the titration in Figure 15-2. (a) Write a balanced titration reaction. (b) Write two different half-reactions
Question:
Consider the titration in Figure 15-2.
(a) Write a balanced titration reaction.
(b) Write two different half-reactions for the indicator electrode.
(c) Write two different Nernst equations for the cell voltage.
(d) Calculate E at the following volumes of Ce4+: 10.0, 25.0, 49.0, 50.0, 51.0, 60.0, and 100.0 mL. Compare your results with Figure 15-2.
Figure 15-2
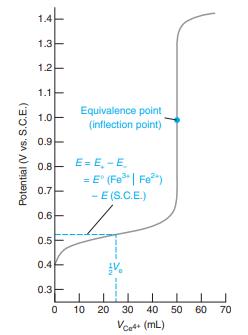
Transcribed Image Text:
1.4 1.3 1.2 1.1 Equivalence point (inflection point) 1.0 0.9 0.8- E= E, -E = E" (Fe| Fe") - E (S.C.E.) 0.7 0.6- 0.5 0.4 0.3 10 20 30 40 50 60 70 Vcet+ (mL) Potential (V vs. S.C.E.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 56% (16 reviews)
a b c d Ce4 Fe2 Ce Fe Fe3 efe2 Eo 0767 V Ce4eCe3 E 170 V E 07670059 16 log 100 mL E ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Chemical Engineering questions
-
Consider the titration of 50.0 mL of 1.0 M glycine hy-drochloride [(H3NCH2COOH)CI], with 1.0 M NaOH. For +H3NCH2COOH, Ka for the carboxylic acid group is 4.3 X 10-3 and Kb, for the amino group is 6.0...
-
Consider the titration of a generic weak acid HA with a strong base that gives the following titration curve: On the curve indicate the points that correspond to the following. a. the equivalence...
-
Consider the titration of 100.0 mL of 0.10 M phosphoric acid with 0.10 M NaOH. a. Determine the pH at the third half- equivalence point by assuming it is a special point (see Fig.). b. Calculate the...
-
Draw the BST that results when you insert the keys E A S Y QUE S T I O N in that order into an initially empty tree. What is the height of the resulting BST?
-
Swenson Company has the following payroll procedures. (a) Supervisor approves overtime work. (b) The human resources department prepares hiring authorization forms for new hires. (c) A second payroll...
-
What are the uses of profits for health care organizations?
-
Explicar la importancia de la novedad en productos y servicios nuevos, as como la relacin del consumidor en el aprendizaje de estas novedades, en cuanto a su grado de involucramiento.
-
1. How would you describe the culture at BMW? 2. What model of leadership is illustrated at BMW? How does this impact BMWs culture? 3. Using the concepts illustrated in the job characteristics model,...
-
Golden, Inc. has been manufacturing 5,000 units of Part 10541, which is used in one of its products. At this level of production, the unit product cost of Part 10541 is as follows: Direct Materials...
-
What is incremental validity? Does this term apply to the assessment of personality traits?
-
A solution containing 50.0 mL of 0.100 M EDTA buffered to pH 10.00 was titrated with 50.0 mL of 0.020 0 M Hg(ClO 4 ) 2 in the cell shown in Exercise 14-B: S.C.E. 7 titration solution | Hg(l) From the...
-
Why don't Cr 3+ and TiO 2+ interfere in the analysis of Fe 3 + when a Walden reductor, instead of a Jones reductor, is used for prereduction?
-
In the diagram at the right, P is the centroid of RST. If JR = 25, find JS and RS.
-
(AVR) PR=IAVR=1R = (power dissipated by a resistor) (28.12) R
-
As a manager and an entrepreneur, you will face a new challenge - business venture structured on the theory of the firm. You are opening a restaurant in your selected town in the State of NY (please...
-
Install on ubuntu , please provide a screenshot for each step 1)How to install base64 on ubuntu 2)What kind of analysis is performed by Cuckoo? How to install Cuckoo on ubuntu?
-
rt a letter to Rose McBride. Writing Plan - Refusal to a Request Rubric Buffer: Start with a neutral statement on which both reader and writer can agree, such as a compliment, appreciation, a quick...
-
FACTS: The Budvar Company sells parts to a foreign customer on December 1, Year 1, with payment of 20,000 crowns to be received on March 1, Year 2. Budvar enters into a forward contract (with a...
-
Identify three questions an owner might ask and seek to answer during the feasibility stage of a new housing development project.
-
The graph of an equation is given. (a) Find the intercepts. (b) Indicate whether the graph is symmetric with respect to the x-axis, the y-axis, or the origin. -3 6 -6 3 x
-
A method to measure soluble organic carbon in seawater includes oxidation of the organic materials to CO2 with K2S2O8, followed by gravimetric determination of the CO2 trapped by a column of...
-
How many milliliters of 2.15% alcoholic dimethylglyoxime should be used to provide a 50.0% excess for Reaction 26-7 with 0.9984g of steel containing 2.07 wt% Ni? Assume that the density of the...
-
Twenty dietary iron tablets with a total mass of 22.131 g were ground and mixed thoroughly. Then 2.998 g of the powder were dissolved in HNO3 and heated to convert all iron into Fe3+. Addition of NH3...
-
Diplomatic Security Service provides Airport Transportation and Surveillance Service to Foreign Diplomats in Guyana. The company has two support departments - Information Systems and Equipment...
-
Q1: A disparity of bargaining power between the parties to a contract may result in unfair terms but a court is not likely to consider the contract unconscionable. Group of answer choices a. True b....
-
Life Tool Manufacturing has a system in place to recall products that prove to be dangerous at some time after manufacture and distribution. This represents which element of the due care theory?...

Study smarter with the SolutionInn App


