Convert the following Fischer projections into tetrahedral representations, and assign R or S stereochemistry toeach: H (b)
Question:
Convert the following Fischer projections into tetrahedral representations, and assign R or S stereochemistry toeach:
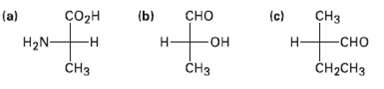
Transcribed Image Text:
сHз (b) (a) H2N- сорн -н сно (c) -Он —он -сно Н CH-CHз CHз CHз т
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 71% (14 reviews)
Horizontal bonds of Fischer projections point o...View the full answer

Answered By

Charles Okinda
students should give all the instructions concerning the challenge that they face. they will get an immediate response because I am always online.
4.90+
754+ Reviews
1483+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Convert the following Fischer projections to perspective formulas. (a) (b) (c) (d) H,N- H- CH;
-
Name and assign R or S stereochemistry to the product(s) you would obtain by reaction of the following substance with ethyl magnesium bromide. Is the product chiral? Is it optically active? Explain.
-
Assign R or S stereochemistry to the chirality centers in the following Newmanprojections: CI (a) (b) C "CH . "
-
Krell Industries has a share price of $22 46 today. If Krell is expected to pay a dividend of S0.83 this year, and its stock price is expected to grow to $24.11 at the end of the year, what is...
-
If you were a team leader at a summer camp for children with special needs, should you include this in your employment history if you are applying for work that is unrelated? Explain your answer.
-
A pressurized tank of water has a 10-cm-diameter orifice at the bottom, where water discharges to the atmosphere. The water level is 2.5 m above the outlet. The tank air pressure above the water...
-
What evidence is there, in this case, of quid pro quo harassment?
-
The following are several situations involving compound interest. Required Using the appropriate table, solve each of the following: 1. Hope Dearborn invests $40,000 on January 1, 2007 in a savings...
-
1) Calculate the sales price variance for the scenario mentioned below. We budgeted to sell 50,000 units for $875,000. We actually sold 55,000 units for $923,000. 2) Calculate the operating income...
-
A firm needs to fully satisfy the demand, which is fixed to 900 units and generates unit revenue of $10. However, its production is subject to the random yield. That is, only a random percentage of...
-
Classify each of the following monosaccharide?s: la) 0 (c) (b) (d) CH- . H- C=0 C=0 -- -- -- -- -- -- -- -- -- H2OH -- CH- Ribulose CH Threose CH- Tagatose 2-Deoxyribose
-
Which of the following Fischer projections of glyceraldehydes represent the sameenantiomer? CH- - 3 - -CH- - - CH2 A
-
(a) What specific recommendations would you give the Johnsons for selecting checking and savings accounts that will enable them to effectively use the first and second tools of monetary asset...
-
A jury of 12 is to be created from a pool of 20 men and 10 women. What is the probability that all 12 on the jury will be men?
-
Reproduced below from Farthington Supply's accounting records is the accounts receivable subledger along with selected general ledger accounts. Dec. 31/19 Balance Credit sales in 2020 General Ledger...
-
Linear Correlation Coefficient In Exercises 9-12, the linear correlation coefficient r is provided. Use Table 2-11 to find the critical values of r. Based on a comparison of the linear correlation...
-
Problem 5-4A Adjusting entries and multi-step income statement-perpetual LO5 Use the unadjusted trial balance of Electric Bike on December 31, 2020. Cash Accounts receivable Merchandise inventory...
-
Only Brakes Inc. is a start-up company that raised the following debt capital in its first year: notes payable of $10,000,000; long-term bank debt of $35,000,000; and bonds payable of $60,000,000....
-
Give examples of how Nadia went about increasing her power.
-
Pedro Bourbone is the founder and owner of a highly successful small business and, over the past several years, has accumulated a significant amount of personal wealth. His portfolio of stocks and...
-
Find the pH of a 0.100 M NaCHO 2 solution. The salt completely dissociates into Na + (aq) and CHO 2 (aq), and the Na + ion has no acid or base properties.
-
How does the resonance model for benzene explain the fact that there are only three isomers of dibromobenzene?
-
Which compound is more reactive toward electrophilic substitution (for example, nitration)? OCH or b. CH,CH3 a. ETor
-
The explosive TNT (2,4,6-trinitrotoluene) can be made by nitrating toluene with a mixture of nitric and sulfuric acids, but the reaction conditions must gradually be made more severe as the nitration...
-
XF Ltd. Is an expanding private company in the electric trade. Accounts preparing in January 2019 included the following information: Profit Statement for the year ended 31 st December 2018 Kshs.000...
-
Check On June 15, 2021, Sanderson Construction entered into a long-term construction contract to build a baseball stadium in Washington D.C., for $340 million. The expected completion date is April...
-
Q.1 Bassem Company purchased OMR420,000 in merchandise on account during the month of April, and merchandise costing OMR $350,000 was sold on account for OMR 425,000. Required: 1. Prepare journal...

Study smarter with the SolutionInn App


