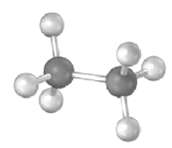
Question: Convert the following representation of ethane, C2H6. Into a conventional drawing that uses solid, wedged, and dashed lines to indicate tetrahedral geometry around each carbon
Convert the following representation of ethane, C2H6. Into a conventional drawing that uses solid, wedged, and dashed lines to indicate tetrahedral geometry around each carbon (gray = c, ivory =H).
Step by Step Solution
3.52 Rating (169 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
22-C-O-C (203).docx
120 KBs Word File


