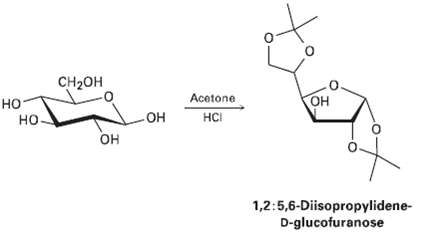
D-Glucose reacts with acetone in the presence of acid to yield the non-reducing 1, 2: 5, 6-diisopropylidenc-D-glucofuranose.
Question:
D-Glucose reacts with acetone in the presence of acid to yield the non-reducing 1, 2: 5, 6-diisopropylidenc-D-glucofuranose. Propose amechanism.
Transcribed Image Text:
Cн2он Acetone Он но HCI HO но- он 1,2:5,6-Diisopropylidene- D-glucofuranose it
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 88% (9 reviews)
Glucopyranose is in equilibrium with glucofuranose Reaction with t...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Glucose reacts with ammonia in the presence of a trace of acid to give predominantly -D- glucopyranosylamine. Propose a reasonable mechanism for this transformation. Why is only the hydroxy group at...
-
In a sulfuric acid plant, sulfur is burned in the presence of excess oxygen to produce sulfur dioxide, (SO,) which in turn is further reacted in the next step with oxygen in a converter to produce...
-
In the presence of light, chlorine can substitute for one (or more) of the hydrogens in an alkane. For the following reactions, draw the possible monochlorination products. hr 2,2-dimethylpropane Cl2...
-
Why does Sam Goldman go out of his way to talk about the importance of d.lights business plan? In what ways do you think having a meticulously crafted business plan helped d.light in its launch...
-
What are some questions to ask when gauging the audience's needs during the planning of a persuasive message?
-
What is the effect of a quota of 13 thousand gallons of water per month on the opportunity set of the consumer in Solved Problem 4.2?
-
Outdoor Greenery, owned 60 percent by Kwasi Peterson and 40 percent by Maya Jefferies, grows specimen plants for landscape contractors. The wholesale price of each plant is $15. During the past year,...
-
Refer to the facts in Exercise. Assume that both Building A and Building B work on just two jobs during the month of June: RW-12 and RW-13. Costs are allocated to jobs based on labor-hours in...
-
Power Company issued a $1,000,000, 5%, five-year bond payable at face value on January 1, 2024. Interest is paid semiannually on January 1 and July 1. Requirements 1. Journalize the issuance of the...
-
The following is the statement of financial position of TT and Co. (see Self-assessment question 3.1 on page 105) at the end of its first year of trading: During 2010, the following transactions took...
-
Iso trehalose and neotrehalose are chemically similar to trehalose except that neotrehalose is hydrolyzed only by -glycosidase enzymes, whereas iso trehalose is hydrolyzed by both -and-glycosidase...
-
D-Mannose reacts with acetone to give a diisopropylidene derivative that is still reducing toward Tollens reagent. Propose a likely structure for this derivative.
-
The separate condensed balance sheets of Patrick Corporation and its wholly owned subsidiary, Sean Corporation, are as follows: Additional Information: On December 31, 2023, Patrick acquired 100...
-
IRIS Ratio Analysis Spreadsheet Using the information presented below, calculate the ratios requested in the IRIS ratio section. All of your answers should be in percentage-format. Note that the...
-
https://www.youtube.com/watch?v=jQbXao0mQ1M 1. Identify the cultural misunderstandings that occurred during Kenichi Takahashi's meeting with Rob, Ella, and Stephanie. Explain and support your...
-
Using what you know of groups and teams, how do you get your teams back on track with Pat? What actions do you need to take as a leader to minimize/repair the negative impact Pat's has had on the...
-
The demand function for a certain product is given by p = 3000 2x + 100 (0 x 10) where x (measured in units of a thousand) is the quantity demanded per week and p is the unit price in dollars. Sketch...
-
QUESTION 4 Dr. Martin Luther King's Speech where he proclaimed, "Free at last! Free at last!" is an example of O A. Impromptu O B. Dissolving OC. Prepared D. Reference E.Planned Speaking Wording...
-
WHAT IS MIS?
-
In Problem use absolute value on a graphing calculator to find the area between the curve and the x axis over the given interval. Find answers to two decimal places. y = x 3 ln x; 0.1 x 3.1
-
A mixture of NaCN and NaHSO 4 consists of a total of 0.60 mol. When the mixture is dissolved in 1.0 L of water and comes to equilibrium, the pH is found to be 9.9. Find the amount of NaCN in the...
-
Using the peroxy acid epoxidation of an alkene and the ring opening of an epoxide, devise a two-step synthesis of 1, 2-butanediol from 1-butene.
-
Write an equation for the reaction of ethylene oxide with a. 1 mole of HCl b. Excess HCl c. Phenol + H+ d. Phenylmagnesium bromide
-
CH3CH2OCH2CH2OH (ethyl cellosolve) and CH3CH2OCH2CH2OCH2CH2OH (ethyl carbitol) are solvents used in the formulation of lacquers. They are produced commercially from ethylene oxide and certain other...
-
44. Dryer Companys policy is to keep 25% of the next month's sales in ending inventory. If Dryer meets its ending inventory policy at the end of April and sales are expected to be 24,000 units in May...
-
What general conclusions can you draw about your companys liquidity, solvency and productivity based on your ratio calculations. Working Capital 2017 = $9,994 M 2016 = $10,673 M Current Ratio 2017 =...
-
Tami Tyler opened Tami's Creations, Incorporated, a small manufacturing company, at the beginning of the year. Getting the company through its first quarter of operations placed a considerable strain...

Study smarter with the SolutionInn App


