Does ascorbic acid (vitamin C) have a D or Lconfiguration? HO . C=0 Ascorbic acid . -H
Question:
Does ascorbic acid (vitamin C) have a D or Lconfiguration?
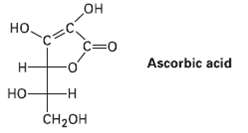
HO но. C=0 Ascorbic acid Н. -H но- CH2OH
Step by Step Answer:
Ascorbic acid has an L configurat...View the full answer



Related Video
Lemon juice preserves apples by slowing down the oxidation process. Oxidation is a chemical reaction that occurs when oxygen reacts with certain substances, such as apples. When an apple is cut or bitten, oxygen is exposed to the inside of the apple and causes enzymes in the apple to turn brown, which is an indication of oxidation. The browning process is caused by the production of polyphenol oxidase (PPO) enzymes that convert phenolic compounds into quinones, which then polymerize to form the brown pigments. One of the compounds present in lemon juice is ascorbic acid (vitamin C), which is a natural antioxidant. Antioxidants work by neutralizing the free radicals that cause oxidation. When lemon juice is applied to apples, the ascorbic acid in the lemon juice reacts with the PPO enzymes and slows down the browning process. You can do an experiment by cutting apples into small pieces, leaving one apple piece in contact with air and the others covered with lemon juice and compare the browning process. This will help to understand the antioxidation process in fruits.
Students also viewed these Organic Chemistry questions
-
Ascorbic acid (vitamin C) does not contain a traditional carboxylic acid group, but it is, nevertheless, still fairly acidic (pKa = 4.2). Identify the acidic proton, and explain your choice using...
-
Ascorbic acid, or vitamin C(C 6 H 8 O 6 ), is an essential vitamin. It cannot be stored by the body and must be present in the diet. What is the molar mass of ascorbic acid? Vitamin C tablets are...
-
A 3.87-mg sample of ascorbic acid (vitamin C) gives 5.80 mg CO2 and 1.58 mg H2O on combustion. What is the percentage composition of this compound (the mass percentage of each element)? Ascorbic acid...
-
A factory with three departments uses a single production overhead absorption rate expressed as a percentage of direct wages cost. It has been suggested that departmental overhead absorption rates...
-
Spurred in part by the success of numerous do-it-yourself (DIY) TV shows, homeowners across the country are redecorating, remodeling, and rebuilding. Many people are content with superficial changes,...
-
Choose the best answer. 1. Which of the following would not be considered a general long-term liability? a. The estimated liability to clean up the hazardous waste storage sites of the citys Public...
-
Magnum Manufacturing rewards its key executives exclusively on return on investment (ROI). The vice president of administration suggests to the CEO that Magnum can increase its ROI by outsourcing...
-
EVA is used by top management at College Learning Technologies to measure and evaluate the performance of segment managers. The company's cost of capital is 11 percent. In 2010, its Audio/Visual...
-
(4 Task 3: Marks) Casio Travels forecasts to pay a OMR (last two digits of your student number) dividend next year, which represents 100% of its earnings. This will provide investors with a 12%...
-
The data for the number employed at several famous IT companies is maintained in the COMPANY table. Write a query to print the /Ds of the companies that have more than 10000 employees, in ascending...
-
Write open-chain structures for the following: (a) A ketotetrose (b) A ketopentose (c) A deoxyaldohexose (d) A five-carbon amino sugar
-
Draw the three-dimensional furanose form of ascorbic acid (Problem 25.32) , and assign R or S stereochemistry to each chirality center. . c=0 Ascorbic acid - CH2
-
If you buy our airline ticket now you can save 60 percent, and that means 60 percent more vacation for you. Most of the following selections were taken from letters-to-the-editor columns of news...
-
2. Getting ready for Logarithms and Calculus! a. Fill in the chart and graph the function (I advise practicing on your scientific calculator and desmos. X f(x) = Inx 0 0.5 1 e 10...
-
JoJo Co. had the following balances and information for October. Beg. finished goods inventory = $30 Beg. work in process inventory = $5 Beg. raw materials inventory = $15 End. finished goods...
-
Subway sales have been declining since 2014. In the US, Subway has closed a number of stores due to over-expansion, outdated operations, and uninspiring menus. In Canada, Subway took a different...
-
Harvey Auto Parts purchased a new crane on September 1 for $35,000, paying $10,000 cash and signing a 7%, 12-month note for the remaining balance, interest to be paid at maturity. The crane is...
-
e4(k+1) Find the sum of the series. k = 1 8
-
Do you think servant leadership generally works well in hospitality organizations? In some departments better than others? Explain.
-
Refer to Example 9.15. Add the following functionality to this program: Allow the user to enter the cost of a gallon of gas on each trip and use a function, Cost() to calculate the cost of purchasing...
-
Find the pH of a 0.200 M HNO 2 solution.
-
Provide equations for the synthesis of the following compounds from 1-bromo-1-phenylethane. OCH2CH N(CH3)2 a. b. C. d. e.
-
Devise a synthesis of a. CH3OCH2CH2CH3 from an alkoxide and an alkyl halide. b. CH3OC(CH3)3 from an alcohol and an alkyl halide.
-
Provide an equation for the preparation of the following alkene from an alkyl halide. Do you anticipate problems with formation of other elimination or substitution reactions? Explain.
-
How does the life time analysis differ from the basic customer profitability approach(500 words)
-
The security analysis research reports, published by sell side analysts working for security firms and FINRA, purport to sell an analyst's investment ideas to an investor in exchange for...
-
During the month of September,the Cider Pressing Company is trying to determine how much cider they are going to sell in October and November. One gallon of cider typically sells for $7 per gallon....

Study smarter with the SolutionInn App


