Draw the two chair conformations of menthol, and tell which is morestable. C Menthol CH(CH3)2
Question:
Draw the two chair conformations of menthol, and tell which is morestable.
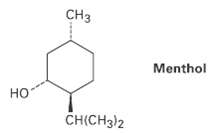
Transcribed Image Text:
Cнз Menthol но CH(CH3)2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 58% (12 reviews)
From the flatring drawing you can see that the methyl group and the OH group have a cis relation...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Draw the two chair conformations of the sugar α-(+)-glucopyranose, one form of the sugar glucose. Which of these two forms is the major one at equilibrium? Explain. CH2OH OH ...
-
Draw the two chair conformations of cis-1-chloro-2-methylcyclohexane. Which is more stable, and by how much?
-
Draw the two chair conformations of trans-1-chloro-2-mcthylcyclohexane. Which is more stable?
-
A strange function. Consider McCarthys 91 function: public static int mcCarthy(int n) { if (n > 100) return n - 10; return mcCarthy(mcCarthy(n+11)); } Determine the value of mcCarthy(50) without...
-
Tammy Jackson purchased 100 shares of All-American Manufacturing Company stock at $31.50 a share. One year later, she sold the stock for $38 a share. She paid her broker a $15 commission when she...
-
Just months into her new job as CEO, Marissa Mayer ended Yahoos telecommuting policy, sparking a lively debate in the national media. A company wide memo instructing employees to return to the...
-
What are five basic components of an accounting system? AppendixLO1
-
Recently the high and low market prices of Canadian Pacific Limiteds debentures were $790 and $475, respectively. Determine the yield-to-maturity of one of these debentures if it was purchased under...
-
Klein Company distributes a high-quality bird feeder that sells for $65 per unit. Variable costs are $26 per unit, and fixed costs total $180,000 annually. Required: Answer the following independent...
-
1. Should you discuss the matter first with Troy before responding to Joyce? Explain. 2. Assume Kristen is a Certified Management Accountant and member of the Institute of Management Accountants. As...
-
Galactose, a sugar related to glucose, contains a six-membered ring in which all the substituents except the ?OH group indicated below in red are equatorial. Draw galactose in its more stable chair...
-
There are four cistransisomers of menthol (Problem 4.37), including the one shown. Draw the other three.
-
When a self-employed individual travels for business but mixes in some pleasure days, such as sightseeing, how does the taxpayer deduct expenses connected with the travel expenses to and from the...
-
4. What is the time complexity of the following procedure for in/2 to n do j 2 end for while (j
-
If the concentration of a constituent in the influent to the equalization basin is constant over the 24 h period, will the load of the constituent from the basin be constant? If the concentration of...
-
A three-phase transmission line of a 60 Hz circuit has a length of 370 km (230 miles). the conductors are of the 795,000cm (54/7) type with horizontal spacing of 25 feet between them. The load on the...
-
Simulate rolling a dice using Math.random() . Your roll function should allow the caller to specify any number of sides, but default to 6 if no side count is given: roll() assumes a 6 sided dice,...
-
Drama Read the excerpt from a play. Then, answer the question(s). (1) (2) Belle: Having trouble deciding what will make you look like both a power to be reckoned with and a fetching young lady while...
-
Understand the differences between observation and interview methods.
-
Briefly describe the following types of group life insurance plans: a. Group term life insurance b. Group accidental death and dismemberment insurance (AD&D) c. Group universal life insurance d....
-
Arrange the following substances in the order in which you would expect their boiling points to increase: CCl 4 , Cl 2 , ClNO, N 2 .
-
Provide a mechanism for the following reaction, based on your knowledge of the reaction of esters with Grignard reagents. OH MgBr (2 equiv.) Cl (2) NH,CI
-
Provide retrosynthetic analyses and syntheses for each of the following alcohols, starting with appropriate alkyl or aryl halides. (a) (b) (c) (d) (e) (f) OH (three ways) OH (three ways) (two ways)...
-
Consider the allylic bromination of cyclohexene labeled at C3 with 13C. Neglecting stereoisomers, what products would you expect from this reaction? NBS, ROOR heat (-13C-labeled position)
-
Your firm is planning to invest in an automated packaging plant. Harburtin Industries is an all - equity firm that specializes in this business. Suppose Harburtin ' s equity beta is 0 . 8 7 , the...
-
Ned Allen opened a medical practice in Los Angeles, California, and had the following transactions during the month of January. (Click the icon to view the January transactions.) Journalize the...
-
do you need more information or are you working on this? Irene Watts and John Lyon are forming a partnership to which Watts will devote one- half time and Lyon will devote full time. They have...

Study smarter with the SolutionInn App


