Each of the following compounds has a single 1H NMR peak. Approximately where would you expect each
Question:
Each of the following compounds has a single 1H NMR peak. Approximately where would you expect each compound toabsorb?
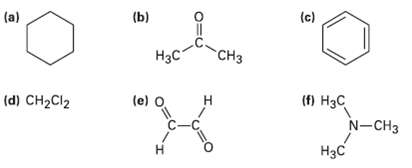
Transcribed Image Text:
(c) (b) (a) Нас "СHз (d) CH2CI2 (e) O (п Нас N-CHз н C-C Нзс н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 73% (15 reviews)
Compound a C6H12 b CH3COCH 3 c C6H6 d CHCl ...View the full answer

Answered By

Vineesh kumar V
To work in your esteemed organization where I can prove my expertise and work towards the growth of the organization
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Each of the following compounds has a nitrogennitrogen bond: N2, N2H4, N2F2. Match each compound with one of the following bond lengths: 110 pm, 122 pm, 145 pm. Describe the geometry about one of the...
-
Each of the following compounds has been prepared from p-nitroaniline. Outline a reasonable series of steps leading to each one. (a) p-Nitrobenzonitrile (d) 3, 5-Dibromoaniline (b) 3, 4,...
-
Each of the following compounds has been prepared from o-anisidine (o-methoxyaniline). Outline a series of steps leading to each one. (a) o-Bromoanisole (d) 3-Fluoro-4-methoxybenzonitrile (b)...
-
According to Thomson Financial, last year the majority of companies reporting profits had beaten estimates. A sample of 162 companies showed that 114 beat estimates, 29 matched estimates, and 19 fell...
-
How would you characterize the nature of competition in the restaurant industry? Are there submarkets with distinct competitive pressures? Are there important substitutes that constrain pricing?...
-
You are given the equation(s) used to solve a problem. For each of these, a. Write a realistic problem for which this is the correct equation(s). b. Finish the solution of the problem. |(9.0 x 10...
-
What is meant by the equivalence of the unit of analysis? Explain with examples.
-
Ramrod, Inc., sells a warehouse for $350,000. It purchased the warehouse 10 years ago for $250,000 and had taken $75,000 in depreciation on the building to the date of sale. Identify the tax issue(s)...
-
Question 9 20 pts Floral Prints Inc. is considering the purchase of a new silkscreen machine. The cost information of the current machine and the new are presented below. Current Machine New Machine...
-
Use the following data to calculate the variances in the following questions. Standard Cost Profile Lab Treatment SU #12 Expected Treatments= 1,000 Standard Cost Profile Lab Treatment SU #12 Expected...
-
How many absorptions would you expect S)-malate, an intermediate in carbohydrate metabolism, to have in its NMR spectrum?Explain. (S)-Malate
-
Identify the different kinds of non-equivalent protons in the following molecule, and tell where you would expect each toabsorb. CH-CH C
-
It is a law of nature that the total energy of the universe is conserved. What do politicians mean, then, when they urge "energy conservation"?
-
Name and define the more common constraints in any given project.
-
Graph the function f(x)=-x+4x-20 State where f(x) is increasing and decreasing. State any absolute extrema (if they exist). Determine the Domain and Range.
-
A residential wiring circuit is shown in the figure. In thismodel, the resistor R 3 is used to model a 250 V appliance(such as an electric range), and the resistors R 1 and R 2 are used to model 125...
-
1. The speed limit on some interstate highways is roughly 100 km/h. (a) What is this in meters per second? (b) How many miles per hour is this? 2. A car is traveling at a speed of 33 m/s. (a) What is...
-
Questions 33 and 34 are based on the following information: Bilog Company's budgeted fixed overhead costs are P50,000 and mthe variable factory overhead rate is P4 per direct labor hour. The standard...
-
Are employees being managed to maximize their productivity as well as commitment to the success of the restaurant?
-
In the operation of an automated production line with storage buffers, what does it mean if a buffer is nearly always empty or nearly always full?
-
Calculate the osmotic pressure of a solution containing 24.6 g of glycerin (C 3 H 8 O 3 ) in 250.0 mL of solution at 298 K.
-
Unlike ethylene glycol, propylene glycol (propane-1,2-diol) is nontoxic because it oxidizes to a common metabolic intermediate. Give the structures of the biological oxidation products of propylene...
-
Predict the major products of the following reactions. (a) Ethyl tosylate + potassium tert butoxide (b) Isobutyl tosylate + NaI (c) (R) 2 hexyl tosylate + NaCN (d) The tosylate of cyclohexlmethanol +...
-
Complete the following conversion table. 1700 1640 1600 400 T(cm ) A() 2.50 3.03 3.33 4.55 4000 25.0
-
explain in excel please For a particular product the price per unit is $6. Calculate Revenue if sales in current period is 200 units. Conduct a data analysis, on revenue by changing the number of...
-
Hall Company sells merchandise with a one-year warranty. In the current year, sales consist of 35,000 units. It is estimated that warranty repairs will average $10 per unit sold and 30% of the...
-
Q 4- Crane Corporation, an amusement park, is considering a capital investment in a new exhibit. The exhibit would cost $ 167,270 and have an estimated useful life of 7 years. It can be sold for $...

Study smarter with the SolutionInn App


