Each of the following reaction schemes contains one or more flaws. What is wrong in each case?
Question:
Each of the following reaction schemes contains one or more flaws. What is wrong in each case? How would you correct eachscheme?
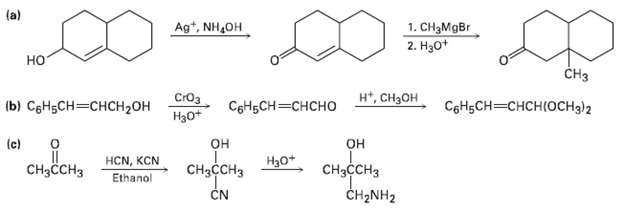
Transcribed Image Text:
(a) Ag*, NH,OH 1. CH3MgBr 2. Нао* но CHз н, Снзон СIОз b) СоНьСH — снсH2он СоНьCH—снсно Нао* CоHgCH—снсHIOснз)2 (c) он CнзссHз CH2NH2 он HCN, KCN Ethanol Нао* CнзссCHз CHзсCHз CN
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
a Basic silver ion does not oxidize secondary alcohol...View the full answer

Answered By

Sheikh Muhammad Ibrahim
During the course of my study, I have worked as a private tutor. I have taught Maths and Physics to O'Level and A'Level students, as well as I have also taught basic engineering courses to my juniors in the university. Engineering intrigues me alot because it a world full of ideas. I have passionately taught students and this made me learn alot. Teaching algebra and basic calculus, from the very basics of it made me very patient. Therefore, I know many tricks to make your work easier for you. I believe that every student has a potential to work himself. I am just here to polish your skills. I am a bright student in my university. My juniors are always happy from me because I help in their assignments and they are never late.
4.90+
14+ Reviews
24+ Question Solved
Related Book For 

Question Posted:
Related Video
In this video, A mixture of methanol and air in a large polycarbonate bottle is ignited. The resulting rapid combustion reaction, often accompanied by a dramatic ‘whoosh’ sound and flames, demonstrates the large amount of chemical energy released in the combustion of alcohol
Students also viewed these Organic Chemistry questions
-
Each of the following independent examples involves one or more of the four major activities carried out by managers at Sights and Sounds, a manufacturer of high-quality televisions and audio...
-
How many signals would the product of the following reaction show in these spectra a. Its 1H NMR spectrum b. Its 13C NMR spectrum 1. excess CH3MgBr 32. H30 CH CCH,CH,COCH
-
Each of the following independent situations has one or more control activity weaknesses. 1. Board Riders Ltd. is a small snowboarding club that offers specialized coaching for snowboarders who want...
-
What is the MFD? UFD? How are they related?
-
As the case study indicates, there appears to be a lack of clear communication occurring between the middle managers and top management. The company's future growth and development depends on clear...
-
A 4.50-kg block of ice at 0.00C falls into the ocean and melts. The average temperature of the ocean is 3.50C, including all the deep water. By how much does the melting of this ice change the...
-
Types of literature review.
-
The Income Summary and Levi Simmons, Capital accounts for Simmons Production Company at the end of its accounting period follow. Complete the following statements: 1. Total revenue for the period is...
-
PLS answer as much as you can no enough remaining thank you so much Beta is a measure of a stock's: (select all that apply) Responsiveness to changes in a market index. Diversifiable Risk Total Risk...
-
Watch the attached video from CBS News titled, Toxins in Baby Food. Assume that you are the newly-hired marketing manager for a baby food company. You have been hired to help your company repair its...
-
The amino acid methionine is biosynthesized by a multistep route that includes reaction of an imine of pyridoxal phosphate (PLP) to give an unsaturated imine, which then reacts with cysteine. What...
-
6-Methyl-5-hepten-2-one is a constituent of lemongrass oil. How could you synthesize this substance from methyl4-oxopentanoate? CH3CH2CH2OCH3 Methyl 4-oxopentanoate
-
The study reports that r = 0.4. What does this mean in plain English? A researcher conducts a study to determine if there is an association between time spent in solitary confinement and depression...
-
Primare Corporation has provided the following data concerning last month's manufacturing operations. Purchases of raw materials Indirect materials used in production Direct labor Manufacturing...
-
1. Start with the temperature at 1000 K. In the data table below, record the peak wavelength. Then, increase the temperature by 1000 K up to 10000 K and record the peak wavelength. Temperature (K)...
-
PROUT COMPANY AND SUBSIDIARYConsolidated Statements WorkpaperFor the Year Ended December 31, 2025Prout SextonEliminationsNoncontrollingConsolidatedCompanyCompanyDebitCreditInterestBalancesINCOME...
-
It is know that 4000 automobile trips are generated in a large residential area from noon to 1:00 P.M. on Saturdays fro shopping purposes. Four major shopping centers have the following...
-
dy 1. Find and simplify. dx tanx (a) y= (3 marks) (b) y x cosh (In x) (3 marks) (c) + sinh 2y = y - cosh 2x (4 marks)
-
Which statement most accurately describes how criteria are established for use by internal auditors in determining whether goals and objectives have been accomplished? (a) Management is responsible...
-
Nitrogen monoxide reacts with hydrogen as follows: 2NO(g)+ H2(g) N2O(g) + H2O(g) The rate law is [H2]/ t = k[NO]2[H2], where k is 1.10 107 L2/(mol2s) at 826oC. A vessel contains NO and H2 at...
-
One of the allotropes of phosphorus consists of four phosphorus atoms at the corners of a tetrahedron. Draw a Lewis structure for this allotrope that satisfies the octet rule. The P 4 molecule can be...
-
Predict the products from the following reactions. (a) (b) (c) (d) (1) 2 exces) NaoH, Ho (2) H,o KOH 2 HOEIOH KOH H2O/EtOH (1) LDA (1.1 equiv) (3) H2o
-
The mandibular glands of queen bees secrete a fluid that contains a remarkable compound known as "queen substance." When even an exceedingly small amount of the queen substance is transferred to...
-
What products would you expect to obtain from each of the following crossed Claisen condensations? (a) (b) Ethyl propanoate+ (1) NaOEt (2) H,o yl oxalate (1) NaOEt Ethyl acetate ethyl formate (2) H,O
-
help!!! Use the above information to calculate ending inventory using FIFO for a company that uses a perpetua/inventory system
-
Rocky Mountain Chocolate Factory (RMCF) founder and president Frank Crail employs 220 people in 361 outlets in the United States, Canada, United Arab Emirates, Japan, South Korea and Saudi Arabia. If...
-
The market price of a semi-annual pay bond is $979.86. It has 21.00 years to maturity and a yield to maturity of 7.34%. What is the coupon rate? Submit Answer format: Percentage Round to: 0 decimal...

Study smarter with the SolutionInn App


