Each of the following syntheses requires more than one step. How would you carry themout? (a) CH3CH2CH2C=CH
Question:
Each of the following syntheses requires more than one step. How would you carry themout?
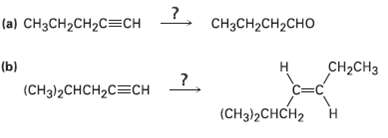
Transcribed Image Text:
(a) CH3CH2CH2C=CH CH3CH2CH2CHO (b) (CHз)2CHCH2CСH CH-CHз н C=C (CHд)2CHCH2 ?. Н
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 53% (15 reviews)
a b H Lindlar catalyst CH3CHCH...View the full answer

Answered By

Deepak Pal
Hi there! Are you looking for a committed, reliable, and enthusiastic tutor? Well, teaching and learning are more of a second nature to me, having been raised by parents who are both teachers. I have done plenty of studying and lots of learning on many exciting and challenging topics. All these experiences have influenced my decision to take on the teaching role in various capacities. As a tutor, I am looking forward to getting to understand your needs and helping you achieve your academic goals. I'm highly flexible and contactable. I am available to work on short notice since I only prefer to work with very small and select groups of students. Areas of interest: Business, accounting, Project management, sociology, technology, computers, English, linguistics, media, philosophy, political science, statistics, data science, Excel, psychology, art, history, health education, gender studies, cultural studies, ethics, religion. I am also decent with math(s) & Programming. If you have a project you think I can take on, please feel welcome to invite me, and I'm going to check it out!
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How would you carry out the following conversions? More than one step is needed in eachcase. "
-
Syntheses of each of the following compounds have been reported in the chemical literature. Using the indicated starting material and any necessary organic or inorganic reagents, describe short...
-
Syntheses of each of the following compounds have been reported in the chemical literature. Using the indicated starting material and any necessary organic or inorganic reagents, describe short...
-
Spencer Company sells lamps and other lighting fixtures. The purchasing department manager prepared the following inventory purchases budget. Spencers policy is to maintain an ending inventory...
-
In the previous problem, suppose that vendor A provides a new price-discount schedule for component 3. This one is an "incremental" discount, as opposed to an "all-units" discount, as follows: Unit...
-
Find the difference in pressure between tanks A and B, if d 1 = 2 ft, d 2 = 6 in., d 3 = 2.4 in., and d 4 = 4 in. Air. B da 45 H20 Hg dz A d1 d2
-
Project Scope Management Create a WBS for this project and enter the tasks in Project 2010. Create milestones and summary tasks. Assume that some of the project management tasks you need to do are...
-
For each of the following situations, explain how risk of material misstatement should be assessed and what effect that assessment will have on detection risk. a. Johnson, Inc., is a fast- growing...
-
Partner Industries sells a single product for $45 that has a variable cost of $35. Fixed costs amount to $5 per unit when anticipated sales targets are metthe company sells one unit in excess of its...
-
Pick an organization (public or private) and then choose a particular type of COI that is associated with this type of organization. Sustainability-related COIs are especially encouraged. Locate and...
-
Propose structures for hydrocarbons that give the following products on oxidative cleavage by KMnO4 orO3: (b) .Co2 (a) O2 + CH(CH2)5C02H CC2H + e) O2CICH2)8CO2H (d) CH + 2H2cO2H + co2 (e) CO2...
-
How would you carry out the following transformation? More than one step isneeded. CH3CH2CH2CH2C=CH T ? CCH2CH2CH2 H
-
Following the financial statements in an annual report is what is called the "Notes to Consolidated Financial Statements." These notes are an important part of the financial statements and can help...
-
3 Below is financial information for December Inc., which manufactures a single product: 4 5 5 7 3 #units produced October Low activity November High activity 7,000 11,000 Cost of goods manufactured...
-
(b) The satellite's booster rockets fire and lift the satellite to a higher circular orbit of radius 2R1. The satellite follows the path shown in the diagram below, moving a total distance S during...
-
D. An airplane flies at a speed of 250 kilometers per hour (kph) at an altitude of 3000 m. Assume the transition from laminar to turbulent boundary layers occurs at critical Reynolds Number, RE cr, =...
-
1. (35 points) by Qet = QoQ = Q0Q2 N!37 N/A (1-B, (r), where A- B(T) V * 4R (e-(R)/KT - 1) R dR is the second virial coefficient. The classical partition function for an imperfect gas comprising N...
-
es Farm has 28 employees who are paid biweekly. The payroll register showed the following payroll deductions for the pay period ending March 23, 2021. Gross Pay 85,950.00 EI Premium Income Taxes...
-
Give some differences between a daily event and a special event. LO.1
-
What will be the final value of DI after executing the following piece of code? Execute the instructions dependently one after another. CLD MOU CX,OFOH MOU AX.02874H MOU DI,01000H MOU ES, DI SUB...
-
If 123 mL of a 1.1 M glucose solution is diluted to 500.0 mL, what is the molarity of the diluted solution?
-
Compound X, isolated from lanolin (sheep's wool fat), has the pungent aroma of dirty sweatsocks. A careful analysis showed that compound X contains 62.0% carbon and 10.4% hydrogen. No nitrogen or...
-
For each of the following structures, 1. Draw a Lewis structure; fill in any nonbonding electrons. 2. Calculate the formal charge on each atom other than hydrogen. All are electrically neutral except...
-
1. From what you remember of electronegativities, show the direction of the dipole moments of the following bonds. 2. In each case, predict whether the dipole moment is relatively large...
-
A project with an initial cost of $32,000 is expected to provide cash flows of $12,900, $13,100, $16,200, and $10,700 over the next four years, respectively. If the required return is 8.1 percent,...
-
A company that is expecting to receive EUR 500,000 in 60 days is considering entering into an FX futures contract to lock an exchange rate to USD for the transaction. The FX rate on the contract is...
-
Suppose you bought a bon with an annual coupon rate of 6.5 percent one year ago for $1,032. The bond sells for $1,020 today. a. Assuming a $1,000 face value, what was your total dollar return on this...

Study smarter with the SolutionInn App


