How would you carry out the following transformation? More than one step isneeded. CH3CH2CH2CH2C=CH T ? CCH2CH2CH2
Question:
How would you carry out the following transformation? More than one step isneeded.
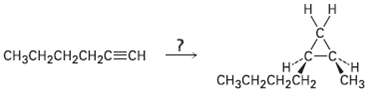
Transcribed Image Text:
нн CH3CH2CH2CH2C=CH Tн н? CнзCH2CH2CH2 СHз
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 72% (18 reviews)
Strategy The product contains a cisdisubstituted cyclopropane ring which can be formed from ...View the full answer

Answered By

Nazrin Ziad
I am a post graduate in Zoology with specialization in Entomology.I also have a Bachelor degree in Education.I posess more than 10 years of teaching as well as tutoring experience.I have done a project on histopathological analysis on alcohol treated liver of Albino Mice.
I can deal with every field under Biology from basic to advanced level.I can also guide you for your project works related to biological subjects other than tutoring.You can also seek my help for cracking competitive exams with biology as one of the subjects.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
How would you carry out the following conversions? More than one step is needed in eachcase. "
-
How would you carry out the following reactions? More than one step may be required. (a) 3-Hexyne 3-Hexanone (b) Benzene m-Bromo acetophenone (c) Bromobenzene Acetophenone (d) 1-Methylcyclohexene ...
-
How would you carry out the following transformations? Co .CO2H (a) (b) Co CH2 C (c) CH2SH
-
Top executive officers of Preston Company, a merchandising firm, are preparing the next years budget. The controller has provided everyone with the current years projected income statement. Current...
-
Universal Technologies, Inc. has identified two qualified vendors with the capability to supply certain of its electronic components. For the coming year, Universal has estimated its volume...
-
A differential manometer is used to measure the pressure change caused by a flow constriction in a piping system as shown. Determine the pressure difference between points A and B in psi. Which...
-
Project Time Management a. Enter realistic durations for each task and then link appropriate tasks. Be sure that all tasks are linked in some fashion to the start and end of the project. Use the...
-
A partnership has assets of $210,000 and liabilities of $95,000. The capital information for the current partners is as follows: Given the above information, respond to each of the following...
-
Question Management accounting has evolved over the years to provide information for determining the cost of product and services and financial control; management planning and control, reducing...
-
Create an application, using the following names for the solution and project, respectively: Glasgow Solution and Glasgow Project. Save the application in the VB2015\Chap11 folder. Create the...
-
Each of the following syntheses requires more than one step. How would you carry themout? (a) CH3CH2CH2C=CH CH3CH2CH2CHO (b) (CH)2CHCH2CH CH-CH C=C (CH)2CHCH2 ?.
-
Synthesize the following compounds using 1-butyne as the only source of carbon, along with any inorganic reagents you need. More than one step may be needed. (a) 1, 1, 2, 2-Tetrachlorobutane (b) 1, 1...
-
Toni Graff, Ahmad Nu, and Lindsay Pane are partners in Travel Essentials. Their partnership agreement stated that withdrawals would be 10 percent for each partner based on the partnerships net...
-
PART 4.1 Process Costing - Weighted Average MOLDING Physical Flow of Units Work-in-Process - Beginning Units Started this Period Units to Account for Total transferred out Work-in-Process - Ending...
-
3. A boy walks 10 m north then 3 m west. What is his total displacement? [3 marks] 4. A and B are perpendicular vectors. A = 2 and A + B a. Calculate b. Calculate A - B c. Explain your results. B bd....
-
Virginia has just been quoted what appears to be a very competitive loan for $2,000 to be paid back in 24 monthly payments of $96.66.What is Virginia's APR? What is the monthly payment for a $24,000,...
-
A four-lane urban freeway (two lanes in each direction) is located on rolling terrain and has 12-ft lanes, no lateral obstructions within 6 ft of the pavement edges, and an interchange every 2 miles....
-
In January, 1993, there were about 1,313,000 internet hosts. During the next five years, the number of hosts increased by about 100% per year. a. Write a model giving the number h (in millions) of...
-
Name the stages in the event planning process. LO.1
-
Find the area of the surface generated by revolving the para- metric curve x = cos 1, y = sin? 1 (0 < I sa/2) about the y-axis.
-
A chemist wants to make 5.5 L of a 0.300 M CaCl 2 solution. What mass of CaCl 2 (in g) should the chemist use?
-
Determine whether the following pairs of structures are actually different compounds or simply resonance forms of the same compounds. (a) (b) (c) (d) (e) (f) (g) (i) (j) and O- and and O- CH C H and...
-
Draw the important resonance forms to show the delocalization of charges in the following ions. (a) (b) (c) (d) (e) (f) (g) (h) (i) (j) CH-C CH H-C-CH CHCH CH2 NH CHCH- CH CH CH-CH 3 CH3 CH CH CH CH...
-
All of the following compounds can react as acids. Without using a table of acidities, rank them in order of increasing acidity. Explain your ranking. (a) CH3CH2SO3H (b) CH3CH2OH (c) CH3CH2COOH (d)...
-
1. Compute the productivity profiles for each year. If required, round your answers to two decimal places. 2a. Did productivity improve? 2b. Explain why or why not
-
Explain: An office building is renting for $10/sf, with 50,000 total leasable square feet. Office buildings in the area are selling for cap rates of 5.5%. What information do you have and what are...
-
Practicum Co. pad $1.2 million for an 80% interest in the common stock of Sarong Co. Practicum had no previous equity interest in Sarong. On the acquisition date, Sarong's identifiable net assets had...

Study smarter with the SolutionInn App


